Abstract
Objective of the study is to compare the diagnostic accuracy for detecting monosodium urate crystal deposits between dual-energy CT (DECT) and ultrasound (US). Sixty consecutive patients (49 men, mean age 62 years) with clinically suspected gout were included in this case–control study. DECT and US of feet, knees, hands and elbows were performed in all patients. Polarisation microscopy of synovial fluid or a score incorporating serum uric acid level, first MTP joint involvement, gender, previous patient-reported arthritis attack, cardiovascular diseases, joint redness and onset within 1 day was used as standard of reference. Standard of reference classified 39 patients as gout positive. Sixteen patients had gout and a concomitant rheumatic disease. Sensitivities for diagnosis of gout disease were 84.6 % (33/39) for DECT and 100 % (39/39) for US. Specificities were 85.7 % (18/21) for DECT and 76.2 % (16/21) for US. Positive and negative predictive values were 91.7 % (33/36) and 75.0 % (18/24) for DECT, 88.6 % (39/44) and 100 % (16/16) for US, respectively. Urate crystals were detected most frequently in MTP1 joints (DECT 20/78, US 58/78), any other toe joints (DECT 25/78, US 62/78) and knees (DECT 41/78, US 31/78). The volumetry of DECT computed a mean urate crystal deposit load of 2.1 cm3 (SD 9.6 cm3). A mean effective dose of ≤0.5 mSv was estimated. DECT is more specific for the diagnosis of gout than US. However, it fails to detect small urate crystal deposits. It might be particularly useful for patients with ambivalent findings, concomitant rheumatic diseases and with non-conclusive joint aspiration.
Similar content being viewed by others
Introduction
The scientific interest in gout has tremendously increased. There is a trend to more obesity and higher age in most populations, and the prevalence of gout has increased to far above 1 % in most countries. New drugs have been approved within the last years, and others are being investigated [1]. Study results have led to profound changes in treatment recommendations [2]. New insights are arising for hyperuricaemia and gout being risk factors for cardiovascular disease and nephropathy [3, 4].
Detection of monosodium urate crystals by polarisation microscopy has been the gold standard for the diagnosis of gout. However, aspiration including microscopy is not always successful. In primary care, joint aspiration is only performed in 3 % of patients [5]. Ultrasound (US) and dual-energy computed tomography (DECT) are new diagnostic tools providing fascinating images particularly in patients with severe gout [6–11]. How do they perform in clinical practice for differentiating gout from other inflammatory diseases? What is their role in patients with gout and concomitant rheumatic disease?
The aim of this study was to compare the diagnostic accuracy of DECT and US for detecting monosodium urate crystal deposits with reference to the clinical diagnosis of gout.
Materials and methods
Study design
This case–control study was approved by an institutional review board. Inclusion criteria were as follows: (1) clinical suspicion of gout by the attending rheumatologist; (2) patient available for musculoskeletal US examination and DECT; (3) attempt made to receive material for polarisation microscopy; and (4) signed patient informed consent for using the medical data for research purposes.
Patient population
Between August 2011 and March 2012 patients undergoing DECT according to a gout protocol were included. DECT for the identification of gout is a Food and Drug Administration (FDA)-approved technique and was offered as a diagnostic option for patients who presented in a tertian rheumatological centre with the clinical suspicion of gout with or without another inflammatory rheumatic disease. Differential diagnoses included particularly seronegative rheumatoid arthritis (RA), psoriatic arthritis (PSA), osteoarthritis and other crystal diseases such as chondrocalcinosis (CPPD) and hydroxyapatite deposition disease (HADD).
The following features were assessed for every patient:
-
History or clinical aspect of podagra.
-
Attempt for polarisation microscopy of joint or tophus aspirate.
-
Highest known urate concentration in serum.
-
Arterial hypertension or cardiovascular diseases.
-
Previous patient-reported arthritis attack, onset within 1 day, joint redness.
-
US and DECT of both feet, knees, hands and elbows.
Computed tomography
Dual-energy CT (DECT) (Somatom Definition Flash; Siemens Healthcare, Forchheim, Germany) was performed in dual-energy mode with tube potentials of 80 kV (tube A) and 140 kV (tube B). A thin filter in the high-energy beam was used to increase the separation of the two X-ray spectra. The acquisition parameters were a slice collimation of 40 × 0.6 mm, slice width 0.75 mm, pitch of 0.7, reference mAs of 250 mAs (tube A) and 125 mAs, individual effective mAs based on the automated dose modulation algorithm, reconstructed slice width of 0.75 mm, and a kernel D30f. The feet, knees and hands were scanned bilaterally, and each elbow was scanned separately. All scans were obtained without intravenous contrast agent. The feet and the knees were scanned in supine position. The other acquisitions were performed in prone position.
The reconstructed low- and high-energy images were analysed by a radiologist (AH, 10 years experience in musculoskeletal imaging) using a commercially available software tool (Gout, Syngo CT Workplace; Siemens Healthcare). For each voxel, the CT numbers in the low- and high-energy images represent the amount of X-ray attenuation that occurred within that voxel. A fully automated material decomposition algorithm for identifying monosodium urate crystal and calcium voxels according to their material-specific dual-energy behaviour was used. The tophus volume or urate deposit load by examination region was calculated (Volume, Siemens Healthcare).
Radiation exposure
Dedicated software (CT-Expo V2.0 2010, Medical University Hannover, Germany) was used for calculation of effective doses [12]. The software takes into account the patient gender, scanner type and the overranging effects of multislice CT. For the calculation, the dose-length products (DLPs) of the feet and the knees of each patient computed by the scanner were used. The effective dose takes into account the distribution of dose amongst the radiosensitive organs into the body by summing the individual organ doses, having weighted each one according to the relative sensitivity of the organ to radiation-induced somatic or genetic effects.
Ultrasound
Musculoskeletal US was performed by a rheumatologist (20 years of experience in musculoskeletal US) with a MyLab Twice system (Esaote SpA, Milan, Italy) using a 6–18 MHz probe for hands, feet and elbows and a 3–9 MHz probe for the knees. The joints and the tendons were examined circumferentially in long and short axis.
At least one of the following two findings had to be present in order to classify a patient as US-positive for gout:
-
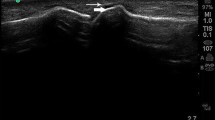
Intraarticular hyperechoic, inhomogeneous material with a cloudy appearance with or without partial or complete posterior shadowing representing intraarticular gout tophi (Fig. 1b).
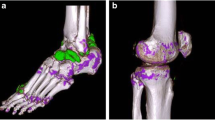
Fig. 1 Gout of left MTP1 joint. An 82-year-old male with urate crystal deposits proven by polarisation microscopy. a DECT in three-dimensional volume-rendering reconstruction technique depicts extensive urate crystal deposits (green colour mapping; arrows) in the MTP1 joints and around the extensor tendons. Artefacts in the nails can be seen (asterisk). The volume urate crystal load in both feet was 4.7 cm3. b Longitudinal medial US of the left MTP1 joint confirms the CT findings with intraarticular hyperechoic inhomogeneous material (asterisk) and “double contour sign” (arrows). c DECT reformatted in sagittal plane depicts multiple urate crystal deposits in the left lateral MTP1 joint (arrows). Note the clearly lower spatial resolution of CT
-
Abnormal hyperechoic band over the superficial margin of the articular hyaline cartilage, which is independent of the angle of insonation, representing crystal deposition on the surface of the cartilage (Fig. 1b).
If one of these findings were present in at least one joint region, other US findings were regarded as positive for gout:
-
Hyperechoic, more homogeneous intraarticular material (Figs. 2c, 3b).
Fig. 2 HADD of right MCP2 joint. A 51-year-old male with false-positive US findings. a Before utilisation of the material decomposition algorithm, CT reformatted in the coronal plane reveals intraarticular high density material (arrow). b After use of the material decomposition algorithm, calcified material typical for HADD (arrow) is depicted. c US with longitudinal-lateral acquisition of the right MCP2 joint depicts intraarticular hyperechoic material (arrows) that cannot been differentiated from other crystal deposits
Fig. 3 Gout of left MTP5 joint. A 45-year-old male with urate crystal deposits proven by aspiration in the left MTP5 joint. a DECT depicts a small tophus in the left MTP 5 joint in three-dimensional volume-rendering reconstruction technique (arrow). An artefact is seen in the nail of the first toe (asterisk). b A longitudinal-lateral US scan of the left MTP5 joint also shows the abnormalities (arrow) including hyperechoic cloudy interarticular material and double contour sign. The power Doppler signals represent the hypervascularisation caused by the acute inflammation. c Images reformatted in the coronal plane from the foot. DECT depicts a singular urate crystal deposit (arrow)
-
Extraarticular hyperechoic, inhomogeneous material with a cloudy appearance with or without partial or complete posterior shadowing, potentially surrounded by a small anechoic or hypoechoic rim representing extraarticular gout tophi.
Analysis
Patient-based evaluation
Sensitivity and specificity analyses for DECT and US were performed against a final rheumatological diagnosis as a standard of reference (SOR). The SOR was rated positive in case of detection of monosodium urate crystals using a polarisation microscope with compensator or for a Janssens score ≥8 [13]. The score (maximum 13 points) was used in all patients without detection of urate crystals by microscopy and incorporates the following variables: serum uric acid level exceeding 350 μmol/l (3.5 points), first metatarsophalangeal joint (MTP) involvement (2.5 points), gender (male, 2 points), previous patient-reported arthritis attack (2 points), arterial hypertension or one or more cardiovascular diseases (1.5 points), joint redness (1 point) and onset within 1 day (0.5 points). The readers of DECT and US were blinded to the results of the other imaging modality and to the final diagnosis. The final diagnosis was established on the basis of microscopy or a positive score including a rheumatologist who did not perform imaging independent of the results of DECT or US.
Joint-based evaluation
The joint regions listed in Table 1 were evaluated bilaterally by DECT and US. All examinations were done within less than 2 weeks from each other. Detection of urate crystal deposits by DECT and US was evaluated bilaterally for the following evaluation units in all patients with the final diagnosis gout: MTP1 joint, any other toe joints, midfoot/ankle, knee, fingers, wrist and elbow.
Statistical analysis
Continuous data were shown as means with standard deviation and compared between patients with and without final diagnosis gout by a Wilcoxon rank sum test. Categorical data were presented as absolute and relative frequencies. The 95 % confidence intervals for sensitivity, specificity and positive and negative predictive value on a patient basis were calculated based on the Clopper–Pearson approach using SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Sixty patients were included in the study (49 males). The mean age was 62 years (SD 11.3, range 36–82 years). The mean body mass index (BMI) was 30.4 kg/m2 (SD 6.1 kg/m2). Thirty-nine patients (65 %) were finally diagnosed with gout according to the SOR. The other patients were finally diagnosed with RA (n = 6), PSA (n = 4), undifferentiated oligoarthritis (n = 3), CPPD, osteoarthritis (n = 2, respectively), ankylosing spondylitis (AS), undifferentiated connective tissue disease, Lyme disease and HADD (n = 1, respectively). No difference in age was found between the groups (p = 0.6250). Of the 39 patients with gout, 16 (41 %) had a concomitant inflammatory rheumatic disease, i.e. RA (n = 9), PSA (n = 3), ankylosing spondylitis (AS), systemic lupus erythematosus (SLE), systemic sclerosis and CPPD (n = 1, respectively).
Of the 39 gout patients, 31 had been newly diagnosed with gout. The eight other patients had been previously diagnosed with gout because of podagra (n = 7) or polyarticular disease (n = 1). Of these eight patients, six received allopurinol, and one received febuxostat. One of these patients and the 31 newly diagnosed patients did not receive any pharmacological urate lowering treatment at the time of evaluation.
We aimed at receiving material from tissue or joint aspirates of every patient in order to perform polarisation microscopy. However, the diagnosis of gout could be confirmed by polarisation microscopy in only 18 of the 39 (46 %) gout patients. In nine patients (23 %), fluid aspiration was successful, but microscopy was negative. In eight patients (21 %), no fluid could be aspirated for further examination (punctio sicca). Aspiration was not done in three gout patients (8 %) because of anticoagulation. One gout patient refused joint aspiration. The 21 patients who were diagnosed as having gout without positive microscopy had a median Janssens score of 11.5 (minimum, 8; maximum, 13). Microscopy was negative in all 10 non-gout patients who were aspirated. In six non-gout patients, no fluid could be aspirated (punctio sicca). Aspiration was not done in three non-gout patients because of anticoagulation. Two non-gout patients refused aspiration.
Of the gout patients, 28 (72 %) had a history of podagra. The mean documented maximum serum uric acid level of was 525 μmol/l in gout patients (minimum–maximum, 251–854 μmol/l) and was found to be significantly higher than in non-gout patients (418 μmol/l, 243–900 μmol/l, p = 0.0061). The highest documented maximum serum uric acid level was above 360 μmol/l in 34 (87 %) of the gout patients and in 12 (57 %) of the non-gout patients. The mean MDRD-GFR (Glomerular Filtration Rate according to the “Modification of Diet in Renal Disease Study”) was 71 ml/min (27–119 ml/min) in gout patients and 85 ml/min (33–135 ml/min) in non-gout patients.
Patient-based evaluation
Dual-energy CT (DECT) had a sensitivity of 84.6 % (33/39 patients, 95 % CI 69.5 %; 94.1 %) for diagnosis of gout disease. US had a sensitivity of 100 % (39/39 patients, 95 % CI 91.0 %; 100 %). The specificity was 85.7 % (18/21 patients, 95 % CI 63.7 %; 97.0 %) for DECT and 76.2 % (16/21 patients, 95 % CI 52.8 %; 91.8 %) for US. The positive predictive value was 91.7 % (33/36 patients, 95 % CI 77.5 %; 98.2 %) for DECT and 88.6 % (39/44 patients, 95 % CI 75.4 %; 96.2 %) for US. The negative predictive value was 75.0 % (18/24 patients, 95 % CI 53.3 %; 90.2 %) for DECT and 100 % (16/16 patients, 95 % CI 79.4 %; 100 %) for US. All patients with a false-positive DECT were negative in US. All patients with a false-positive US had a negative DECT.
When considering only the 18 polarisation microscopy positive gout patients and the 21 patients with other rheumatic diseases, sensitivities were similar, 83.3 % for DECT (15/18 patients) and 100 % for US (18/18 patients). The respective positive predictive values were 83.3 % (15/18 patients) for DECT and 78.3 % for US (18/23 patients). The negative predictive values were 85.7 % for DECT (18/21 patients) and 100 % for US (16 of 16 patients).
Dual-energy CT (DECT) was false-positive in three patients without a history of podagra and with normal uric acid serum levels showing minimal deposits suggestive of gout tophi in the lateral menisci. One patient was finally diagnosed with CPPD (Fig. 4), one with PSA and one with undifferentiated oligoarthritis.
CPPD of the right knee. A 67-year-old male with CPPD deposits at the right lateral knee compartment classified as CT false-positive. a Coronal MIP reconstruction of 80 kV CT dataset reveals hyperdensity of the lateral meniscus (arrow). b DECT in three-dimensional volume-rendering reconstruction technique depicts intramensical urate crystals (arrows). c US with longitudinal acquisition depicts intracartilaginous hyperechoic material (arrow). d DECT in similar plane reveals no intracartilaginous urate crystal deposits (oval contour). The intramensical deposits are depicted (arrow)
Ultrasound (US) was false-positive in five patients. Two patients with double contour sign and hyperechoic material at MCP, PIP and MTP joints had another crystal deposition disease. One patient had CPPD; the other had HADD (Fig. 2). A double contour sign without hyperechoic material in the right MTP1 joint was misinterpreted as gout in an RA-patient. Two patients with severe peripheral arterial occlusive disease and arterial calcifications also displayed intraarticular hyperechoic material. In one patient with PSA, this was localised in the talonavicular and cuneonavicular joints. In another patient with RA, this was localised in MCP and MTP joints.
Dual-energy CT (DECT) was false-negative in six patients. In three patients, US showed typical abnormalities in only one MTP1 joint, in one patient in both MTP 1 joints, in one patient in both MTP1 joints and the left olecranon bursa, and in one patient in one MTP joint and both cuneonavicular joints. All these patients had typical podagra; and polarisation microscopy was positive in three of these patients. Uric acid serum levels were elevated in four patients at the time of the gout attack.
Joint-based evaluation
Detection of urate crystals by evaluation unit is depicted in Table 2. A good correlation for large intraarticular monosodium urate crystal deposits was seen (Figs. 1, 3), whereas for small intraarticular monosodium crystal deposits or joints revealing exclusively, a double contour sign DECT frequently was found to be false-negative.
The ten most frequently involved regions in DECT (according to Table 1) were the quadriceps tendons in 16/39 patients (41 %), the lateral knee joints in 15/39 patients (38.5 %), the medial knee joints and the MTP1 joints (each in 14/39 patients, 35.9 %), the Achilles tendons in 11/39 patients, the crucial ligaments in 10/39 (25.6 %), the patellar tendons in 9/39 (23.1 %), the extensor tendons of the foot, the midfoot joints and the peroneus tendons (each in 8/39 patients, 20.5 %).
Urate volume
The volumetry of all monosodium urate crystal deposits over all examination regions computed a mean crystal load of 2.1 cm3 (SD 9.6 cm3). The mean load was 0.97 cm3 (SD 4.13 cm3) for the feet, 0.42 cm3 (SD 1.77 cm3) for the knees, 0.34 cm3 (SD 1.68 cm3) for the hands and 0.35 cm3 (SD 1.27 cm3) for the elbows.
Radiation exposure
A mean total DLP of 878.3 mGy cm (SD 82.1) was computed, 310.2 mGy cm (SD 21.4) for the feet, 161.1 mGy cm (SD 19.9) for the knees, 186 mGy cm (SD 11.9) for the hands, 120.1 mGy cm (SD 16.2) for the right elbow and 118.9 mGy cm (SD 17.0) for the left elbow. A mean effective dose of 0.1 mSv was estimated for feet and knees. The hands and the elbows in prone “over-head” position cannot be calculated by the software and were estimated with ≤0.1 mSv for every region. Overall, a radiation exposure of ≤0.5 mSv for the five regions of interest was estimated.
Discussion
Dual-energy CT (DECT) has the potential to differentiate gout from other rheumatic diseases, particularly from other crystal diseases such as CPPD and HADD. Images can be easily read, stored and re-evaluated.
The protocol of this study examining feet, knees, hands and elbows bilaterally provides an excellent overview of the most relevant joints combined with a moderate radiation exposition of ≤0.5 mSv. Other anatomical areas can be examined according to particular clinical indications. In selected cases, DECT may help find an adequate site for aspiration.
Our protocol produces around 5,000 images. The in-room time is 10–15 min per patient. Post-processing and evaluation needs another 30 min. The DECT technology is complex and currently limited to high-end CT with two tubes. However, the availability of CT scans providing this technology is increasing worldwide.
Dual-energy CT (DECT) is less sensitive than US on a joint-based evaluation due to a lower spatial resolution. The diagnosis of gout may be missed by CT in patients with early disease limited to few joints [11]. DECT is able to delineate intraarticular tophi. However, it misses crystal depositions on the surface of the cartilage, which represent the double contour US sign [14, 15].
Small dots in menisci and nails might be misinterpreted as gout. Otherwise, the advantage of DECT is its high specificity due to its ability to differentiate between different crystals.
The first study using DECT for gout showed impressive images of severe urate crystal deposits [16]. DECT may become a tool for follow-up examinations in selected cases due to its good reproducibility and independency of the examiner [6, 17, 18]. However, DECT will become particularly valuable in patients with ambivalent clinical findings [7] and for patients with gout and concomitant rheumatic diseases. A recent study reveals that tendons are frequently affected by gout [19].
A retrospective study found a sensitivity of 100 % and a specificity of 84 % in a collective of 12 patients with positive and 19 patients with negative polarisation microscopy [8]. Even in specialised centres, aspiration is not always available or successful. Microscopy missed gout crystals in 25 % of successfully aspirated joints [20]. Patients may refuse aspiration. It may not be done because of anticoagulation. Attempts to aspirate fluid particularly from small joints like the MTP1 joints often fail. Urate crystals may localise at the bottom of an effusion. Thus, more superficially located aspirated fluid may be free of crystals. Finally, tophi in the soft tissue, e.g. next to the femoral condyles, may not be connected with the aspirated effusion. Imaging is important for confirming the diagnosis particularly in patients in whom the diagnosis cannot be supported by microscopy [9].
Another study prospectively investigated the diagnostic accuracy of DECT in 80 patients, 40 of whom had a positive polarisation microscopy [16]. The specificity was 93 %. The sensitivity was 78 %. This study evaluated patients with advanced gout. The mean index tophus had a volume of 2.5 cm3, which is more than the mean total tophus volume of our patients (2.1 cm3). The study showed a good reproducibility of DECT. Furthermore, CT proved to be positive also in 11 of 14 patients with early disease proven by microscopy in another study [21].
Ultrasound (US) has been suggested as the imaging method of choice for the diagnosis and management of gout [22–24]. Unfortunately, large variations for the sensitivity in different patient collectives have been observed, depending notably on the severity of gout and, therefore, on the amount of urate crystal deposits [14, 25, 26]. In addition, specificity varies between the different US signs of urate crystal deposition, i.e. the double contour sign, hyperechoic cloudy areas and others like bright aggregates [22, 27]. In fact, the double contour seems to be the most specific sign (up to 99 %) [22, 26] but has a limited prevalence and seems to be associated with longstanding gout [27, 28]. Even an experienced sonographer may, therefore, misinterpret other crystal depositions or arteriosclerosis as gout. CPPD crystals usually localise inside the cartilage. Nevertheless, they may penetrate into the synovium simulating gout. US features for gout are currently defined and tested for reliability by an OMERACT ultrasound subtask force. In clinical practice, US examinations can be done only at selected anatomical regions because of time constrains: recent studies have demonstrated a good balance between sensitivity and specificity of a 6 min US examination protocol including the MTP1’s and knees [27] or of a 12-structure US assessment of one joint (radiocarpal joint), two tendons (patellar tendon and triceps tendon) for hyperechoic aggregates and three articular cartilages (MTP1, talar and MTP2 or femoral condyle) for double contour sign [29]. For the selection, our results confirm the conclusions of Peiteado et al. in their collective but are not fully in accordance with Naredo et al.: we agree that the MCP1 joint is commonly involved. However, gout occurs less frequently at the wrist, particularly at the wrist differentiating between CPPD and gout with US is challenging [27, 29].
Our study had some limitations. This is a retrospective study. Until recently, the diagnosis of gout and another inflammatory rheumatic disease in the same patient was supposed to be extremely rare. However, two studies concluded that the coincidence of both diseases occurs as frequently as statistically expected [30, 31]. The coincidence rate is far higher in this study. All patients were seen in a tertiary rheumatology referral centre with a primary ambivalent diagnosis of gout. Furthermore, several patients had been treated at the author’s institution for an inflammatory rheumatic disease before the first symptoms of gout arose. Such a population will represent a typical population for the future use of DECT in clinical rheumatological practice. Finally, interobserver variability was not evaluated, neither for DECT nor for US.
Dual-energy CT (DECT) for the diagnosis of gout is less sensitive than US due to its inferior spatial resolution but findings are very specific to differentiate urate crystal deposits from other crystals [32]. DECT will not be needed in diagnosis for every patient with suspected gout, but it might be useful particularly for patients with ambivalent findings, with gout and concomitant other rheumatic diseases, and in those many patients whose joint aspiration and microscopy did not successfully detect gout crystals. This study shows that a protocol examining feet, knees, hands and elbows provides a good overview on relevant anatomical structures and that it is feasible in clinical practice.
References
Crittenden DB, Pillinger MH (2013) New therapies for gout. Annu Rev Med 64:325–337
Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T et al (2012) American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 64:1431–1446
Feig DI, Kang DH, Johnson RJ (2008) Uric acid and cardiovascular risk. N Engl J Med 359:1811–1821
Kuo CF, See LC, Luo SF, Ko YS, Lin YS, Hwang JS et al (2010) Gout: an independent risk factor for all-cause and cardiovascular mortality. Rheumatology (Oxford) 49:141–146
Owens D, Whelan B, McCarthy G (2008) A survey of the management of gout in primary care. Ir Med J 101:147–149
McQueen FM, Doyle A, Dalbeth N (2011) Imaging in gout—what can we learn from MRI, CT, DECT and US? Arthritis Res Ther 13:246
Nicolaou S, Liang T, Murphy DT, Korzan JR, Ouellette H, Munk P (2012) Dual-energy CT: a promising new technique for assessment of the musculoskeletal system. AJR Am J Roentgenol 199(Suppl 5):S78–S86
Glazebrook KN, Guimarães LS, Murthy NS, Black DF, Bongartz T, Manek NJ et al (2011) Identification of intraarticular and periarticular uric acid crystals with dual-energy CT: initial evaluation. Radiology 261:516–524
Desai MA, Peterson JJ, Garner HW, Kransdorf MJ (2011) Clinical utility of dual-energy CT for evaluation of tophaceous gout. Radiographics 31:1365–1375
Choi HK, Burns LC, Shojania K, Koenig N, Reid G, Abufayyah M et al (2012) Dual energy CT in gout: a prospective validation study. Ann Rheum Dis 71:1466–1471
Gruber M, Bodner G, Rath E, Supp G, Weber M, Schueller-Weidekamm C (2014) Dual-energy computed tomography compared with ultrasound in the diagnosis of gout. Rheumatology (Oxford) 53:173–179
Stamm G, Nagel HD (2002) CT-expo—a novel program for dose evaluation in CT. Fortschr Röntgenstr 174:1570–1576
Janssens HJ, Fransen J, van de Lisdonk EH, van Riel PL, van Weel C, Janssen M (2010) A diagnostic rule for acute gouty arthritis in primary care without joint fluid analysis. Arch Intern Med 170:1120–1126
Thiele RG, Schlesinger N (2007) Diagnosis of gout by ultrasound. Rheumatology (Oxford) 46:1116–1121
Grassi W, Meenagh G, Pascual E, Filippucci E (2006) “Crystal clear”-sonographic assessment of gout and calcium pyrophosphate deposition disease. Semin Arthritis Rheum 36:197–202
Choi HK, Al-Arfaj AM, Eftekhari A, Munk PL, Shojania K, Reid G et al (2009) Dual energy computed tomography in tophaceous gout. Ann Rheum Dis 68:1609–1612
Dalbeth N, Schauer C, Macdonald P, Perez-Ruiz F, Schumacher HR, Hamburger S et al (2011) Methods of tophus assessment in clinical trials of chronic gout: a systematic literature review and pictorial reference guide. Ann Rheum Dis 70:597–604
McQueen FM, Reeves Q, Dalbeth N (2013) New insights into an old disease: advanced imaging in the diagnosis and management of gout. Postgrad Med J 89:87–93
Dalbeth N, Kalluru R, Aati O, Horne A, Doyle AJ, McQueen FM (2013) Tendon involvement in the feet of patients with gout: a dual-energy CT study. Ann Rheum Dis 72:1545–1548
Swan A, Amer H, Dieppe P (2002) The value of synovial fluid assays in the diagnosis of joint disease: a literature survey. Ann Rheum Dis 61:493–498
Manger B, Lell M, Wacker J, Schett G, Rech J (2012) Detection of periarticular urate deposits with dual energy CT in patients with acute gouty arthritis. Ann Rheum Dis 71:470–472
Mathieu S, Pereira B, Couderc M, Soubrier M (2013) Usefulness of ultrasonography in the diagnosis of gout: a meta-analysis. Ann Rheum Dis 72:e23
Dalbeth N, Doyle A, McQueen FM (2012) Imaging in gout: insights into the pathological features of disease. Curr Opin Rheumatol 24:132–138
Chowalloor PV, Keen HI (2013) A systematic review of ultrasonography in gout and asymptomatic hyperuricaemia. Ann Rheum Dis 72:638–645
Wright SA, Filippucci E, McVeigh C, Grey A, McCarron M, Grassi W et al (2007) High-resolution ultrasonography of the first metatarsal phalangeal joint in gout: a controlled study. Ann Rheum Dis 66:859–864
Filippucci E, Riveros MG, Georgescu D, Salaffi F, Grassi W (2009) Hyaline cartilage involvement in patients with gout and calcium pyrophosphate deposition disease. An ultrasound study. Osteoarthr Cartil 17:178–181
Peiteado D, De Miguel E, Villalba A, Ordóñez MC, Castillo C, Martín-Mola E (2012) Value of a short four-joint ultrasound test for gout diagnosis: a pilot study. Clin Exp Rheumatol 30:830–837
Ottaviani S, Richette P, Allard A, Ora J, Bardin T (2012) Ultrasonography in gout: a case–control study. Clin Exp Rheumatol 30:499–504
Naredo E, Uson J, Jiménez-Palop M, Martínez A, Vicente E, Brito E et al (2013) Ultrasound-detected musculoskeletal urate crystal deposition: which joints and what findings should be assessed for diagnosing gout? Ann Rheum Dis 2013 May 14 (Epub ahead of print)
Jabakumar A, Crowson CS, Udayakumar PD, Matteson EL (2012) Co-existence of gout in rheumatoid arthritis: it does happen! A population based study. Arthritis Rheum 64(Suppl):S58
Perez-Ruiz F, Herrero-Beites AM (2012) Prevalence of non-gout arthritis in patients with gout: Not as sparing as previously thought. Arthritis Rheum 64(Suppl):S63
McQueen FM, Doyle A, Reeves Q, Gao A, Tsai A, Gamble GD et al (2014) Bone erosions in patients with chronic gouty arthropathy are associated with tophi but not bone oedema or synovitis: new insights from a 3 T MRI study. Rheumatology (Oxford) 53:95–103
Acknowledgments
The authors especially thank Carina Schuecke for the CT data acquisition and Carsten Schwenke (SCOSSiS Statistical Consulting) for his assistance in the statistical evaluation.
Conflict of interest
Alexander Huppertz is a full-time paid employee of Siemens AG since June 1, 2004. His function is Associate Director of the Imaging Science Institute Charité. The Institute is a scientific cooperation between the Charité, University Hospitals of Berlin, Germany and Siemens Healthcare in form of a private–public partnership (PPP). Wolfgang A Schmidt received research grants from Esaote SpA and General Electrics. All other authors have no competing interests to declare with respect to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huppertz, A., Hermann, KG.A., Diekhoff, T. et al. Systemic staging for urate crystal deposits with dual-energy CT and ultrasound in patients with suspected gout. Rheumatol Int 34, 763–771 (2014). https://doi.org/10.1007/s00296-014-2979-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-014-2979-1