Abstract
Aims/hypothesis
Hyperglycaemia in diabetes is associated with increased glycation, oxidative stress and nitrosative stress. Proteins modified consequently contain glycation, oxidation and nitration adduct residues, and undergo cellular proteolysis with release of corresponding free adducts. These free adducts leak into blood plasma for eventual renal excretion. The aim of this study was to perform a comprehensive quantitative analysis of protein glycation, oxidation and nitration adduct residues in plasma protein and haemoglobin as well as of free adducts in plasma and urine to quantify increased protein damage and flux of proteolytic degradation products in diabetes.
Methods
Type 1 diabetic patients (n=21) and normal healthy control subjects (n=12) were studied. Venous blood samples, with heparin anticoagulant, and 24-h urine samples were taken. Samples were analysed for protein glycation, oxidation and nitration adducts by a quantitative comprehensive screening method using liquid chromatography with triple quadrupole mass spectrometric detection.
Results
In type 1 diabetic patients, the concentrations of protein glycation, oxidation and nitration adduct residues increased up to three-fold in plasma protein and up to one-fold in haemoglobin, except for decreases in pentosidine and 3-nitrotyrosine residues in haemoglobin when compared with normal control subjects. In contrast, the concentrations of protein glycation and oxidation free adducts increased up to ten-fold in blood plasma, and urinary excretion increased up to 15-fold in diabetic patients.
Conclusions/interpretation
We conclude that there are profound increases in proteolytic products of glycated and oxidised proteins in diabetic patients, concurrent with much lower increases in protein glycation and oxidation adduct residues.
Similar content being viewed by others
Introduction
Early-stage adducts of protein glycation, fructosamines, are measured in diabetes as an indicator of glycaemic control [1]. Protein glycation, as well as protein oxidation and nitration, is thought to contribute to vascular cell dysfunction and the development of microvascular diabetic complications (retinopathy, nephropathy and neuropathy) [2–4]. Recent research has shown that protein glycation, oxidation and nitration are increased in cellular and extracellular proteins in diabetes [5]. Cells maintain the quality and functional integrity of proteins by degradation and replacement of damaged proteins; oxidation and glycation are major types of physiological protein damage [5, 6]. Cellular proteolysis liberates the glycated, oxidised and nitrated amino acids as free adducts. These are released into blood plasma and excreted in urine [5]. The changes in plasma concentrations and urinary excretion of glycation, oxidation and nitration free adducts may reflect tissue damage in diabetes and provide new markers indicative of the damaging effects of hyperglycaemia. A comprehensive assessment of these adducts in plasma and urine has not yet been carried out.
Glycation of proteins is a complex series of parallel and sequential reactions collectively called the Maillard reaction. It occurs in all tissues and body fluids. Early-stage reactions lead to the formation of the early glycation adduct fructosyl–lysine (FL) and other fructosamines, and later-stage reactions form AGEs [2]. FL degrades slowly to form AGEs. Glyoxal, methylglyoxal and 3-deoxyglucosone (3-DG) are also potent glycating agents formed by the degradation of glycolytic intermediates, glycated proteins and lipid peroxidation. They react with proteins to form AGEs directly. Important AGEs, in a quantitative sense, are hydroimidazolones derived from arginine residues and modified by glyoxal, methylglyoxal and 3-DG (G-H1, MG-H1 and 3DG-H, respectively). Other important and widely studied AGEs are \(N_{{\text{ $ \varepsilon $ }}} \)-carboxymethyl-lysine (CML), \(N_{{\text{ $ \varepsilon $ }}} \)-carboxyethyl-lysine (CEL) and pentosidine [5]. Major quantitative markers of oxidative damage to proteins are methionine sulphoxide (MetSO) and N-formylkynurenine (NFK), formed by the oxidation of methionine and tryptophan respectively [7, 8], and a widely studied marker of nitration damage to proteins is 3-nitrotyrosine (3-NT) [9] (Fig. 1).
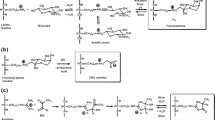
Protein glycation, oxidation and nitration residues. a α-Oxoaldehyde glycating agents. b Early glycation adduct: FL residue. c AGE residues. d Protein oxidation and nitration adduct residues. For the structures of glycation, oxidation and nitration free adducts, those shown in b–d, the terminal amino group is protonated –NH3 + and the terminal carbonyl is a carboxylate –CO2 − moiety
In this paper, we report the comprehensive quantitative analysis of protein glycation, oxidation and nitration adduct residues in plasma protein and haemoglobin, and of related protein glycation, oxidation and nitration free adduct concentrations in plasma, urinary excretion and renal clearance in type 1 diabetic patients with moderate glycaemic control when compared with normal healthy subjects. There were profound increases in the plasma concentration and urinary excretion of selected protein glycation and oxidation free adducts in diabetes.
Subjects and methods
Subjects
The recruitment of diabetic patients for this study has been described previously [10] and patient characteristics are summarised in Table 1. Twenty-one type 1 diabetic patients, with normal creatinine clearance (although three patients had microalbuminuria) and no other microvascular complications, and 12 normal healthy control subjects were recruited. Diabetic subjects received insulin therapy: humulin regular insulin combined with intermediate (NPH or lente) or long-acting (ultralente) insulin. Venous blood samples were taken after overnight fasting with heparin anticoagulant, and 24-h urine samples were collected from diabetic patients and normal healthy control subjects. Blood cells were sedimented by centrifugation and plasma was removed. Urine samples were collected at ambient temperature. Validation studies showed a change of less than 10% in urinary analyte amounts during this period. Plasma and urine samples were stored at −80°C prior to analysis. The study protocol conformed to the ethical guidelines of the latest Declaration of Helsinki and was approved by the local ethics review committee. Informed consent was obtained from all participants.
Measurement of protein glycation, oxidation and nitration adducts by LC-MS/MS
The following glycation adducts were measured: FL, methylglyoxal-derived AGEs (MG-H1, CEL, argpyrimidine and methylglyoxal-derived lysine dimer [MOLD]), glyoxal-derived AGEs (G-H1, CML and glyoxal-derived lysine dimer [GOLD]), 3-DG-derived AGEs (3DG-H and 3-deoxyglucosone-derived lysine dimer [DOLD]) and pentosidine. The protein oxidation adducts MetSO, NFK, dityrosine and 3-NT, and the amino acids lys, arg, met, tyr and trp were also measured. Protein glycation, oxidation and nitration free adducts were measured by assay of analytes in the ultrafiltrate of plasma and urine. Ultrafiltrates were prepared by centrifugation at 4°C through microspin filters (12,000-M r filter cut-off, 50-μl aliquot). Glycation, oxidation and nitration adduct residues of plasma protein and haemoglobin were measured in exhaustive enzymatic digests (50 μg protein equivalent) prepared as described previously (with control subjects for protease autolysis) [11]. Samples were assayed by liquid chromatography with triple quadrupole mass spectrometric detection (LC-MS/MS) with stable isotope-substituted internal standardisation as described previously [5]. Pentosidine, NFK and trp were measured by liquid chromatography with modified fluorimetric detection: the mobile phase was 0.1% trifluoroacetic acid with isocratic 10% acetonitrile from 0 to 20 min and a linear gradient of 10–50% acetonitrile from 20 to 50 min eluted through column 1 only at a flow rate of 0.4 ml/min. The retention time, detection λ excitation/λ emission and limit of detection (LOD) for the analytes were as follows: pentosidine–25.7 min, 320/385 nm and 6 fmol; NFK – 43.7 min, 330/437 nm and 13 pmol; and tryptophan –46.2 min, 286/400 nm and 29 pmol. Authentic standard analytes were prepared as described elsewhere [5, 12].
Other biochemical measurements
HbA1c was measured by the HPLC Diamat method (BioRad, Irvine, CA, USA) [13]. Urinary and plasma creatinine were determined by colorimetric assay (diagnostic kit 510; Sigma). Fasting plasma glucose (FPG), mean daily plasma glucose (24hG) and postprandial changes in plasma glucose (PPG) were assessed as described previously [10].
Statistical analysis
Significance for mean and median analyte values was assessed by Student’s t-test and the Mann–Whitney U-test, respectively. Correlation analysis was performed by calculating Spearman’s rho r statistic. Two-sided p values lower than 0.05 were considered statistically significant.
Results
Measurement of protein glycation, oxidation and nitration adduct residues in plasma protein and haemoglobin and of related free adducts in the blood plasma and urine by LC-MS/MS
Protein glycation, oxidation and nitration adduct residues were detected in hydrolysates of plasma protein and haemoglobin, and corresponding free adducts were detected in the ultrafiltrate of blood plasma and urine. Detection by LC-MS/MS has a high specificity, as there is analyte resolution by chromatographic retention time, molecular mass and fragment mass [5]. Specimen chromatograms of glycation, oxidation and nitration markers are given (Fig. 2a–f), showing the detection of CML free adducts in urine, of MG-H1 and MetSO free adducts in plasma ultrafiltrate, and of 3-NT residues in haemoglobin, reported here for the first time. The detection results for MG-H1 show two partially resolved peaks, as expected, for the racaemic mixture of MG-H1 epimers [5].
Specimen analytical chromatograms of protein glycation, oxidation and nitration adduct residues and free adducts. Analytes and multiple reaction monitoring transitions (molecular ion>fragment ion; M r) are: a CML (204.9>130.1) and b 10 pmol [13C6]CML standard (210.9>136.1) in the urine of a control subject; c MG-H1 free adduct (229.2>114.3) and d 50 pmol [15N2]MG-H1 standard (231.2>116.3) in the plasma filtrate of a diabetic patient; e MetSO free adduct (166.1>102.2) and f 50 pmol [2H3]MetSO standard (169.1>102.2) in the plasma filtrate of a diabetic patient; g 3-NT residues (227.1>181.2) and 10 pmol [2H3]3-NT standard (230.1>184.2) in the haemoglobin of a control subject (50 μg protein equivalent). Chromatographic conditions are described in the Subjects and methods section
Glycation, oxidation and nitration adduct residues in the plasma protein
The concentration of FL residues in the plasma protein was increased by 172% in diabetic patients undertaking regular insulin therapy vs control subjects. The concentration of CML residues was increased by 9%, but not significantly, in diabetic patients vs control subjects, and the concentration of CEL residues was increased by 166%. Hydroimidazolones were major AGE residues in the plasma protein of human subjects [5]. The concentrations of G-H1, MG-H1 and 3DG-H residues were increased significantly in diabetic patients (54, 219 and 28%, respectively) vs control subjects. AGE protein crosslinking was studied by measuring the imidazolium crosslinks GOLD, MOLD and DOLD and the fluorescent crosslink pentosidine. The concentrations of GOLD and DOLD residues were lower than the LOD, and those of MOLD and pentosidine were also very low, approximately 0.001 mmol/mol lys, in the plasma protein. The concentrations of MOLD and pentosidine residues were increased significantly in diabetic patients (five-fold and by 33%, respectively). MetSO and NFK residues were increased four- and two-fold, respectively in the plasma protein of diabetic patients. The concentration of 3-NT residues was low in the plasma protein of control subjects, 0.0006 mmol/mol tyr, and was increased two-fold in diabetic patients (Table 2).
Glycation, oxidation and nitration adduct residues in haemoglobin
The concentration of FL residues in haemoglobin was increased by 133% in diabetic patients vs control subjects. The concentration of CML residues was increased by 6%, but not significantly, in diabetic patients vs control subjects, and the concentration of CEL residues was increased by 30% in diabetic patients. The concentrations of MG-H1 and 3DG-H residues were increased by 31 and 99%, respectively in diabetic patients. The concentrations of GOLD and DOLD residues were lower than the LOD in haemoglobin and those of MOLD and pentosidine were also very low, approximately 0.02 and 0.001% of haemoglobin, respectively. The concentration of MOLD residues was not increased significantly in diabetic patients vs control subjects; unexpectedly, the concentration of pentosidine residues in haemoglobin was decreased by 32% in diabetic patients. The concentration of MetSO residues in haemoglobin was increased by 47% in diabetic patients vs control subjects. The concentration of 3-NT residues in normal control subjects was very low, approximately 0.03% of haemoglobin. Unexpectedly, the concentration of 3-NT residues in haemoglobin was decreased by 59% in diabetic patients vs control subjects (Table 2).
Protein glycation, oxidation and nitration free adducts in the blood plasma and urine
FL free adduct was detected in the plasma of control and diabetic subjects, and the concentration was increased by 84% in diabetic patients. The concentration of CML free adduct in plasma was increased four-fold and the concentration of CEL was increased three-fold in diabetic patients vs control subjects. Hydroimidazolone free adducts are, quantitatively, the major AGE free adducts in the plasma of human subjects [5]. The concentrations of G-H1, MG-H1 and 3DG-H free adducts were increased three-, ten- and two-fold, respectively in diabetic patients vs control subjects. The concentration of MetSO free adduct in plasma was increased five-fold in diabetic patients vs control subjects. The concentrations of GOLD, DOLD, pentosidine, NFK and 3-NT free adducts were lower than the LOD in plasma (Table 3).
Urinary excretion of free adducts
Protein glycation, oxidation and nitration free adducts are excreted in the urine [5]. The urinary excretion of FL free adduct was increased five-fold in diabetic patients vs control subjects. The urinary excretion of CML free adduct was increased two-fold in diabetic patients. Hydroimidazolone free adducts are major AGEs excreted in the urine of human subjects [5]. The urinary excretion of MG-H1 free adduct was increased 15-fold in diabetic patients vs control subjects. The MG-derived AGE argpyrimidine free adduct was also detected in urine. The urinary excretion of argpyrimidine free adduct was increased by only 14% in diabetic patients. The urinary excretion of 3DG-H free adduct was increased two-fold in diabetic patients. The urinary excretion levels of the glycation crosslink MOLD and of pentosidine free adducts were increased by 48% and five-fold, respectively in diabetic patients vs control subjects. MetSO is reduced to methionine by renal MetSO reductase; therefore, the urinary excretion of MetSO in normal healthy control subjects is very low, approximately 0.02 nmol/mg creatinine. This was increased four-fold in diabetic patients. Urinary excretion of NFK free adduct was increased six-fold in diabetic patients vs control subjects. Urinary excretion levels of 3-NT and dityrosine were relatively low in normal control subjects (0.034 and 0.186 nmol/mg creatinine, respectively) and were not changed significantly in diabetic patients (Table 3).
The predicted contribution of plasma protein and haemoglobin degradation to the urinary excretion of protein glycation, oxidation and nitration free adducts was deduced assuming degradation rates of 7 and 3 g/day for plasma protein and haemoglobin, respectively [14, 15]. This indicated that MetSO residues released from albumin and haemoglobin combined were 42-fold higher than those excreted in control subjects, 32-fold higher than those in diabetes. Similarly, FL residues released from albumin and haemoglobin were 122 and 83%, respectively, of the excreted amount in control and diabetic subjects. For AGEs, hydroimidazolone residues released from albumin and haemoglobin were 7 and 51% of urinary excretion, CML and CEL residues were 17 and 3% of the excreted total, and MOLD and pentosidine residues were 31 and 20% of excretion; these values decreased in diabetes. For the NFK and 3-NT residues, free adducts released from albumin and haemoglobin combined were, respectively, 33 and 26% of urinary excreted free adducts; these values decreased in diabetes (Table 3).
The renal clearance of creatinine in the diabetic patients of this study was not significantly different from that of normal healthy control subjects. The renal clearance of FL free adduct, however, was increased approximately two-fold in diabetic patients vs control subjects, to a clearance similar to that of creatinine. This suggests there was decreased reabsorption of FL free adduct in the diabetic patients. Both CML and CEL free adducts had decreased renal clearance in diabetic patients vs control subjects. MG-H1 and 3DG-H free adducts had increased renal clearance levels in diabetic patients vs control subjects, which approached that of creatinine. The renal clearance of MetSO in normal control subjects was very low, approximately 0.2 ml/min, and was not changed significantly in diabetic patients (Table 3).
Homotypic correlation of glycation, oxidation and nitration adducts
Homotypic correlations are correlations of glycation, oxidation and nitration adducts of the same type in the following different locations: plasma protein, haemoglobin and the ultrafiltrate of plasma and urine. In normal control subjects, there were no significant homotypic correlations. In diabetic patients, the concentration of FL residues in plasma protein correlated positively with the urinary excretion of FL (r=0.52, p<0.05), and the concentration of FL residues in haemoglobin correlated positively with the concentration of FL free adduct in plasma (r=0.53, p<0.05). There were no significant homotypic correlations for CML, but for CEL the concentrations of CEL residues in plasma protein and haemoglobin correlated positively (r=0.64, p<0.01). For G-H1, the concentration of G-H1 free adduct in plasma correlated positively with the urinary excretion of G-H1 with borderline significance (r=0.53, p=0.051). For MG-H1, the concentration of MG-H1 residues in plasma protein and haemoglobin correlated positively (r=0.62, p<0.01). For 3DG-H, there was a negative correlation between the concentration of 3DG-H residues in plasma protein and that of 3DG-H residues in haemoglobin (r=−0.45, p<0.05). There was no significant homotypic correlation between pentosidine and MOLD. For oxidative markers, the concentration of 3-NT residues in haemoglobin correlated positively with urinary excretion of 3-NT (r=0.79, p<0.001).
Correlation of indicators of glycaemic control with glycation, oxidation and nitration residues in plasma protein and haemoglobin and with related free adducts in plasma and urine
Many significant correlations between protein glycation and oxidation analytes were found in control subjects, particularly for the urinary excretion of free adducts (Table 4). In diabetic patients, HbA1c correlated positively with FPG and 24hPG, but not with PPG. In plasma protein, the concentration of CML residues correlated positively with HbA1c and 24hG; the concentration of G-H1 residues correlated positively with HbA1c; and the concentration of pentosidine residues correlated positively with FPG. In haemoglobin, the concentration of FL residues correlated positively with HbA1c and FPG; the concentration of CML residues correlated positively with HbA1c; and the concentration of pentosidine residues correlated positively with HbA1c and 24hPG. The concentration of NFK residues correlated negatively with HbA1c and 24hG (Table 5). In plasma, the concentration of FL free adduct correlated positively with FPG but negatively with PPG. The concentration of 3DG-H free adduct correlated positively with PPG with borderline significance. In urine, the urinary excretion of dityrosine free adduct correlated positively with FPG and the urinary excretion of 3DG-H correlated negatively with PPG (Table 6). Other correlations between protein glycation, oxidation and nitration adduct residues and free adducts in diabetic patients are also given in Tables 5 and 6.
Discussion
Protein glycation was originally viewed as a post-translational modification that accumulated mostly on extracellular proteins, with slow AGE formation and accumulation through life. This applies to chemically stable AGEs formed on long-lived proteins but not to short-lived glycation adducts (FL and hydroimidazolones, for example) and all glycation adducts on cellular and short-lived extracellular proteins. Protein turnover by cellular proteolysis is probably the major source of glycation, oxidation and nitration free adducts [5]. A further source is absorption from digested food [16], represented schematically in Fig. 3a. The increased urinary excretion of protein glycation and oxidation free adducts in diabetic patients suggests that the diabetic state is associated with significantly increased damage to proteins by glycation and oxidation, with subsequent cellular proteolysis and free adduct elimination. Protein glycation and oxidation free adducts from food contribute to the background exposure [17], but the diabetic state appears to increase markedly the exposure to protein damage. Glycation of albumin to minimal extents, as found in diabetes in vivo, has no significant effect on albumin half-life [18]. Similarly, little change has been observed in the lifespan of red blood cells (RBCs) in diabetic patients vs control subjects [19]. Accordingly, the changes in glycation, oxidation and nitration adduct residues in plasma protein and haemoglobin in the diabetic subjects of this study are not due to changes in protein substrate turnover but rather to changes in protein modification.
a Biodistribution scheme illustrating flows of formation and removal of protein glycation, oxidation and nitration free adducts. b Schematic diagram showing a cross-section through a blood capillary lumen, illustrating the flows of nitric oxide from the endothelium into the red blood cells and formation of peroxynitrite and 3-NT residues in plasma protein and haemoglobin (Hb)
Early glycation adduct fructosyl–lysine residues in plasma protein and haemoglobin, and related free adduct in plasma and urine
In the plasma protein of diabetic patients, we found increased FL residue concentrations, consistent with concentrations for glycated serum protein previously measured by boronate affinity chromatography [20] and immunoassay [21], and similar to the concentration of FL residues predicted from the kinetics of reactivity of albumin assuming a protein half-life of 20 days [22]. In haemoglobin, early glycation adducts of glucose are conventionally shown as HbA1c [1]. The HbA1c is typically comprised of 60% fructosyl–valine residues (β-val-1) and 40% FL residues (mostly on α-lys-61) [23, 24]. Chromatographic methods for measuring intact HbA1c (such as the Diamat protocol used here) underestimate the absolute concentration of HbA1c by a factor of up to two because of on-column haemoglobin subunit exchange [24]. The LC-MS/MS technique measures the absolute concentration of total FL residues in haemoglobin at all sites (not fructosyl–valine). The HbA1c of the diabetic patients in this study was 7.8±0.9%. The estimate of FL as 8.6% haemoglobin was therefore 55% of the total HbA1c (2×7.8%), slightly higher than the 40% expected. FL free adduct was detected in blood plasma and was increased two-fold in diabetes. It was also excreted in urine with a five-fold increase in diabetes. Estimates reported here are similar to those reported previously [25]. The increased FL urinary excretion in diabetes may underestimate increased formation in diabetes, as FL urinary excretion is metabolised efficiently by fructosamine-3-phosphokinase [26]. FL residues in haemoglobin correlated positively with measures of glycaemic control, HbA1c and 24hG, whereas FL residues in plasma protein correlated negatively with CEL and 3DG-H residues. This may indicate that in the oxidising environment of plasma in diabetes, FL residues are susceptible to degradation to AGEs. The negative correlation between FL free adduct concentration in plasma and PPG may reflect increased oxidative instability of this analyte in postprandial hyperglycaemia. FL free adduct concentration in plasma also correlated positively with FPG.
Advanced glycation endproduct residues in plasma protein and haemoglobin, and related free adducts in plasma and urine
Major AGEs in the plasma protein of control and diabetic subjects were the hydroimidazolones MG-H1 and 3DG-H. In diabetic patients, the largest quantitative increases in protein glycation adduct residues of plasma protein occurred for MG-H1 and 3DG-H. The major site of MG-H1 residue formation in human serum albumin was identified recently as arg-410 [27]. The lack of increase in CML residues in the plasma protein of diabetic patients was unexpected and discordant with the prior report by Schleicher et al. [28], who found increased CML residue concentration. In their study, however, patients with high HbA1c (up to 20% haemoglobin) were recruited. Inspection shows that in patients with an HbA1c lower than 10% in the Schleicher study, there was no significant increase in CML residues. The quantitation of CML was also about four-fold lower than reported here, probably because all of the CML residues in plasma protein were detected by our LC-MS/MS method, whereas immunoassay detects only antibody-accessible CML residues on the surface of plasma proteins.
In haemoglobin, MG-H1 and 3DG-H residues were, again, the major glycation adducts in a quantitative sense. AGEs in haemoglobin, measured previously by immunoassay of unidentified epitopes [29, 30], correlated with HbA1c and probably detected CML [31]. Specific immunoassay of CML residues overestimated the concentration ten-fold [32]. The analogous AGE \(N_{{\text{ $ \alpha $ }}} \)-carboxymethyl-valine residues were quantified in haemoglobin by a gas chromatography–mass spectrometry assay and were approximately 0.06% of haemoglobin [31]. The concentrations of MG-H1 and 3DG-H in haemoglobin were approximately ten-fold higher than the concentrations of CML and CEL residues, and 100- and 500-fold higher than the concentrations of MOLD and pentosidine residues respectively. Formation of 3DG-H in RBCs may be linked to the formation of 3-DG by the deglycating action of fructosamine-3-phosphokinase on HbA1c [26, 33]. Application of matrix-assisted laser desorption ionisation (MALDI) mass spectrometry gave evidence of multiple types of modified haemoglobin in diabetes by molecular mass measurement of haemoglobin subunits [34], consistent with the findings of this study. With MALDI mass spectrometry measurements, the quantitation and molecular identity of the modified protein derivatives is often uncertain (compare with measurements of glycated albumin) [35].
The concentration of CML residues in the plasma protein and haemoglobin of diabetic patients correlated with HbA1c, and CML residues in the plasma protein correlated with 24hG, although the changes in CML residue concentration were too small to be statistically significant vs control values. CML residue concentrations in plasma protein and haemoglobin were also linked to 3DG-H residue concentrations and to pentosidine residue concentration in haemoglobin. CML and CEL residues in plasma protein may be formed from lipid-derived precursors without apparent mediation by glyoxal and methylglyoxal [36].
The most profound effects of diabetes on glycation-related variables were the increases in glycation free adduct plasma concentrations and urinary excretion levels. With no significant increase in CML residues in plasma protein and haemoglobin, plasma CML free adduct was increased four-fold and urinary excretion two-fold. The most marked effect, however, was found for MG-H1 free adduct concentration in the plasma and urinary excretion, which increased ten-fold and 15-fold respectively in diabetic patients. It is expected that these increases relate to increased protein modification and cellular proteolysis in tissues suffering biochemical dysfunction in hyperglycaemia, such as the kidney, retina and peripheral nerve, in which MG-H1 protein residues were increased in experimental diabetic rats [5]. This indicates that diabetes, even when under moderate control, presents a severe insult of protein modification. Increased proteolysis has been found in RBCs and muscle wasting in diabetes [37, 38]. If proteolysis is decreased [39], this may also lead to increased tissue protein AGE residues.
Increased formation of methylglyoxal arises mainly from increased levels of triosephosphates in cells accumulating high levels of glucose in hyperglycaemia [40–43]. This is exacerbated by low glyceraldehyde-3-phosphate dehydrogenase activity [37], particularly when inhibited by poly(ADP ribose) polymerase [44]. Ketone body metabolism may also be involved [45], which may explain why type 1 diabetic patients suffer the highest levels of methylglyoxal [46]. Methylglyoxal crosses cell membranes (probably by passive diffusion of the unhydrated form) [41], so the methylglyoxal that modifies plasma protein is probably cell-derived.
Urinary excretion of glycation free adducts is the major form of elimination of glycation adducts from the body [5]. Glycation free adducts are amino acid derivatives; therefore, their elimination is expected to be controlled by glomerular filtration and active secretion as well as tubular reabsorption by amino acid and organic anion and cation transport proteins in the proximal tubular epithelium (Fig. 3a). The decreased renal clearances of CML and CEL free adducts in diabetic patients may be related to decreased renal organic cation transporter activity [47]. The increased renal clearances of MG-H1 and 3DG-H free adducts in diabetes may be due to increased washout by diuresis. MOLD and pentosidine were quantitatively minor AGE free adducts, but had moderately and markedly increased urinary excretion levels, respectively, in diabetic patients.
The predicted contributions of plasma protein and haemoglobin degradation to the excretion of protein glycation, oxidation and nitration free adducts revealed predicted excess production of MetSO and FL free adducts, consistent with the efficient metabolism of MetSO by MetSO reductase [48] and repair of FL residues and free adducts by fructosamine-3-phosphokinase [26, 33]. NFK and 3-NT are also metabolised to kynurenine [49] and 3-nitro-4-hydroxyphenylacetic acid [50] respectively. The predicted high contribution of plasma protein and haemoglobin turnover to urinary excretion of hydroimidazolone free adducts may suggest there are enzymatic mechanisms to repair or metabolise these adducts too. The marked increase in the urinary excretion of FL, CML, MG-H1 and NFK free adducts, relative to increases in corresponding adduct residues in plasma protein and haemoglobin, may indicate a major formation of glycation and oxidation adduct residues in proteins (with subsequent proteolysis) at sites other than plasma and RBCs. Peptides enriched in glycation, oxidation and nitration adducts were not found in vivo [5], except in portal venous plasma [51], although peptides from incomplete cellular proteolysis have been detected in vivo [52, 53].
Oxidation and nitration adduct residues in plasma protein and haemoglobin, and related free adducts in plasma and urinary excretion
Major oxidation adducts in the plasma protein and haemoglobin of control and diabetic subjects were MetSO and NFK residues. The two- to four-fold increases in the plasma protein of diabetic patients reflect an increasing oxidative environment of the blood plasma in diabetes.
The concentration of 3-NT residues in the plasma protein of diabetic patients has been measured previously by immunoassay [54], but was overestimated ten- to 50-fold; the estimates given here are in agreement with independent, artefact-free estimates by LC-MS/MS [9]. The decrease in 3-NT residue concentration in the haemoglobin and the increase in the plasma protein of diabetic subjects vs control subjects may relate to the compartmentalisation of haemoglobin and peroxynitrite formation. Peroxynitrite forms inside RBCs, with nitric oxide (NO) originating outside RBCs and superoxide originating from intra- and/or extra-RBC sources. Increased peroxynitrite formation in diabetes is linked to the increased production of superoxide in vascular cells [55]. As NO crosses from the endothelium through the plasma, it is intercepted by superoxide with increased efficiency in diabetes, increasing the formation of peroxynitrite and 3-NT in plasma proteins and matrix proteins [55] (Fig. 3b). Less NO then enters RBCs to form 3-NT in haemoglobin. The effects of peroxynitrite, as well as the formation of NO [56], are compartmentalised physiologically in the blood. A similar effect of compartmentalised formation of pentosidine precursor may explain the decreased pentosidine residues of haemoglobin in diabetes.
The five-fold increase in plasma MetSO free adduct and the four- and six-fold increases in urinary excretion of MetSO and NFK free adducts in diabetic patients support the role of increased oxidative damage to proteins, with subsequent proteolysis of the oxidised proteins in diabetes. The urinary excretion of dityrosine was ten-fold higher than that of MOLD and pentosidine. Hence, diabetic patients appear to be exposed to increased protein crosslinking by AGE- and oxidant-mediated mechanisms.
In conclusion, clinical type 1 diabetes under moderate metabolic control is associated with a profound increase in the plasma concentration and urinary excretion of protein glycation and oxidation free adducts, particularly methylglyoxal-derived MG-H1. This may be a marker of tissue damage at sites of development of vascular complications and hence a novel indicator of the pathological effects of hyperglycaemia. The increase in AGE free adducts in IGT, type 2 diabetes and IFG, and its association with the progression to and presence of vascular complications now merit investigation.
Abbreviations
- CEL:
-
\( N_{{\text{ $ \varepsilon $ }}} {\text{ - carboxyethyl - lysine}} \)
- CML:
-
\( N_{{\text{ $ \varepsilon $ }}} {\text{ - carboxymethyl - lysine}} \)
- 3-DG:
-
3-deoxyglucosone
- 3DG-H:
-
3-deoxyglucosone-derived hydroimidazolone, \(N_{{\text{ $ \delta $ }}} \)-(5-hydro-5-(2,3,4-trihydroxybutyl)-4-imidazolon-2-yl) ornithine and related structural isomers
- DOLD:
-
3-deoxyglucosone-derived lysine dimer
- FL:
-
fructosyl–lysine
- FPG:
-
mean fasting plasma glucose concentration
- G-H1:
-
glyoxal-derived hydroimidazolone, \( N_{{\text{ $ \delta $ }}} {\text{ - (5 - hydro - 4 - imidazolon - 2 - yl) ornithine}} \)
- GOLD:
-
glyoxal-derived lysine dimer
- 24hG:
-
mean daily plasma glucose concentration
- LC-MS/MS:
-
liquid chromatography with triple quadrupole mass spectrometric detection
- LOD:
-
limit of detection
- MALDI:
-
matrix-assisted laser desorption ionisation
- MetSO:
-
methionine sulphoxide
- MG-H1:
-
methylglyoxal-derived hydroimidazolone, \(N_{{\text{ $ \delta $ }}} \)-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine
- MOLD:
-
methylglyoxal-derived lysine dimer
- NO:
-
nitric oxide
- NOS:
-
nitric oxide synthase
- NFK:
-
N-formylkynurenine
- 3-NT:
-
3-nitrotyrosine
- PPG:
-
postprandial plasma glucose excursion
- RBCs:
-
red blood cells
References
Manley S (2003) Haemoglobin A(1c)—A marker for complications of type 2 diabetes: the experience from the UK Prospective Diabetes Study (UKPDS). Clin Chem Lab Med 41:1182–1190
Thornalley PJ (1999) Clinical significance of glycation. Clin Lab 45:263–273
Baynes JW, Thorpe SR (1999) Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 48:1–9
Pennathur S, Heinecke JW (2004) Mechanisms of oxidative stress in diabetes: implications for the pathogenesis of vascular disease and antioxidant therapy. Front Biosci 9:565–574
Thornalley PJ, Battah S, Ahmed N et al (2003) Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem J 375:581–592
Goldberg AL (2003) Protein degradation and protection against misfolded or damaged proteins. Nature 426:895–899
Wells-Knecht MC, Lyons TJ, McCance DR, Thorpe SR, Baynes JW (1997) Age-dependent increases in ortho-tyrosine and methionine sulfoxide in human skin collagen is not accelerated in diabetes. J Clin Invest 100:839–846
Geibauf A, van Wickern B, Simat T, Steinhart H, Esterbauer H (1996) Formation of N-formylkynurenine suggests the involvement of apolipoprotein B-100 centered tryptophan radicals in the initiation of LDL lipid peroxidation. FEBS Lett 389:136–140
Gaut JP, Byun J, Tran HD, Heinecke JW (2002) Artifact-free quantitation of free 3-chlorotyrosine, 3-bromotyrosine, and 3-nitrotyrosine in human plasma by electron capture-negative chemical ionization gas chromatography mass spectrometry and liquid chromatography–electrospray ionization tandem mass spectrometry. Anal Biochem 300:252–259
Beisswenger PJ, Wood ME, Howell SK, Touchette AD, O’Dell RM, Szwergold BS (2001) α-Oxoaldehydes increase in the postprandial period and reflect the degree of hyperglycaemia. Diabetes Care 24:726–732
Ahmed N, Argirov OK, Minhas HS, Cordeiro CA, Thornalley PJ (2002) Assay of advanced glycation endproducts (AGEs): surveying AGEs by chromatographic assay with derivatisation by aminoquinolyl-N-hydroxysuccimidyl-carbamate and application to Nɛ-carboxymethyl-lysine- and Nɛ-(1-carboxyethyl)lysine-modified albumin. Biochem J 364:1–14
Simat T, Meyer K, Steinhart H (1994) Synthesis and analysis of oxidation and carbonyl condensation products of tryptophan. J Chromatogr A 661:93–99
HalwachsBaumann G, Katzensteiner S, Schnedl W, Purstner P, Pieber T, WildersTruschnig M (1997) Comparative evaluation of three assay systems for automated determination of hemoglobin A(1c). Clin Chem 43:511–517
Russo LM, Bakris GL, Comper WD (2002) Renal handling of albumin: a critical review of basic concepts and perspective. Amer J Kidney Dis 39:899–919
Cohen RM, Franco RS, Joiner CH (2004) Is poor glycemic control associated with reduced red blood cell lifespan? Diabetes Care 27:1013–1014
Henle T (2003) AGEs in foods: do they play a role in uremia? Kidney Int 63:S145–S147
Ahmed N, Thornalley PJ, Luthen R et al (2004) Processing of protein glycation, oxidation and nitrosation adducts in the liver and the effect of cirrhosis. J Hepatol 41:913–919
Johnson RN, Easdale RW, Tatnell M, Baker JR (1991) Significance of variation in turnover of glycated albumin on indexes of diabetic control. Clin Chim Acta 198:229–238
Sayinalp S, Sozen T, Usman A, Dundar S (1995) Investigation of the effect of poorly controlled diabetes mellitus on erythrocyte life. J Diabetes Complications 9:190–193
Yamamoto Y, Tahara Y, Cha T et al (1989) Radioimmunoassay of glycated serum protein using monoclonal antibody to glucitollysine and Coomassie-brilliant blue-coated polystyrene beads. Diabetes Res 11:45–49
Yatscoff RW, Tevaarwerk GJM, Macdonald JC (1984) Quantification of nonenzymically glycated albumin and total serum-protein by affinity-chromatography. Clin Chem 30:446–449
Baynes JW, Thorpe SR, Murtiashaw MH (1984) Nonenzymatic glycosylation of lysine residues in albumin. Methods Enzymol 106:88–98
Roberts NB, Amara AB, Morris M, Green BN (2001) Long-term evaluation of electrospray ionization mass spectrometric analysis of glycated hemoglobin. Clin Chem 47:316–321
Zhang X, Medzihradszhy KF, Cunningham J et al (2001) Characterization of glycated hemoglobin in diabetic patients: usefulness of electrospray mass spectrometry in monitoring the extent and distribution of glycation. J Chromatogr B 759:1–15
Knecht KJ, Dunn JA, Mcfarland KF et al (1991) Effect of diabetes and aging on carboxymethyllysine levels in human urine. Diabetes 40:190–196
Szwergold BS, Howell S, Beisswenger PJ (2001) Human fructosamine-3-kinase. Purification, sequencing, substrate specificity, and evidence of activity in vivo. Diabetes 50:2139–2147
Ahmed N, Dobler D, Dean M, Thornalley PJ (2005) Peptide mapping identifies hotspot site of modification in human serum albumin by methylglyoxal involved in ligand binding and esterase activity. J Biol Chem 280:5724–5732
Schleicher ED, Wagner E, Nerlich AG (1997) Increased accumulation of the glycoxidation product Nɛ-(carboxymethyl)lysine in human tissues in diabetes and aging. J Clin Invest 99:457–468
Makita Z, Vlassara H, Rayfield E et al (1992) Hemoglobin-AGE: a circulating marker of advanced glycosylation. Science 258:651–653
Turk Z, Mesic R, Benko B (1998) Comparison of advanced glycation endproducts on haemoglobin (Hb-AGE) and haemoglobin A(1c) for the assessment of diabetic control. Clin Chim Acta 277:159–170
Cai J, Hurst HE (1999) Identification and quantitation of N-(carboxymethyl)valine adducts in hemoglobin by gas chromatography/mass spectrometry. J Mass Spectrom 34:537–543
Iwamoto H, Motomiya Y, Miura K, Morisawa M, Yoshimura Y, Maruyama I (2001) Immunochemical assay of hemoglobin with N-epsilon-(carboxymethyl)lysine at lysine 66 of the beta chain. Clin Chem 47:1249–1255
Delpierre G, Vertommen D, Communi D, Rider MH, Van Schaftingen E (2004) Identification of fructosamine residues deglycated by fructosamine-3-kinase in human hemoglobin. J Biol Chem 279:27613–27620
Lapolla A, Tubaro M, Reitano R et al (2004) The complexity of non-enzymatic glycation product sets of human globins. Diabetologia 47:1712–1715
Thornalley PJ, Argirova M, Ahmed N, Mann VM, Argirov OK, Dawnay A (2000) Mass spectrometric monitoring of albumin in uraemia. Kidney Internat 58:2228–2234
Karachalias N, Babaei-Jadidi R, Kupich C, Ahmed N, Thornalley PJ (2005) High dose thiamine therapy counters dyslipidemia and advanced glycation of plasma protein in streptozotocin-induced diabetic rats. Ann NY Acad Sci (in press)
Raghothama C, Rao P (1994) Increased proteolysis of oxidatively damaged hemoglobin in erythrocyte lysates in diabetes mellitus. Clin Chem Acta 225:65–70
Mitch WE, Bailey JL, Wang X, Jurkovitz C, Newby D, Price SR (1999) Evaluation of signals activating ubiquitin–proteasome proteolysis in a model of muscle wasting. Am J Physiol 276:C1132–C1138
Portero-Otin M, Pamplona R, Ruiz M, Cabiscol E, Prat J, Bellmunt MJ (1999) Diabetes induces an impairment in the proteolytic activity against oxidized proteins and a heterogeneous effect in nonenzymatic protein modifications in the cytosol of rat liver and kidney. Diabetes 48:2215–2220
Thornalley PJ (1988) Modification of the glyoxalase system in human red blood cells by glucose in vitro. Biochem J 254:751–755
Phillips SA, Thornalley PJ (1993) Formation of methylglyoxal and d-lactate in human red blood cells in vitro. Biochem Soc Trans 21:163
Phillips SA, Thornalley PJ (1993) The formation of methylglyoxal from triose phosphates. Investigation using a specific assay for methylglyoxal. Eur J Biochem 212:101–105
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820
Du X, Matsumara T, Edelsttein D et al (2003) Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest 112:1049–1057
Reichard GA, Skutches CL, Hoeldtke RD, Owen OE (1986) Acetone metabolism in humans during diabetic ketoacidosis. Diabetes 35:668–674
McLellan AC, Thornalley PJ, Benn J, Sonksen PH (1994) The glyoxalase system in clinical diabetes mellitus and correlation with diabetic complications. Clin Sci 87:21–29
Thomas MC, Tikellis C, Burns WC et al (2003) Reduced tubular cation transport in diabetes: prevented by ACE inhibition. Kidney Int 63:2152–2161
Brot N, Weissbach H (1983) Biochemistry and physiological role of methionine sulfoxide reductase in proteins. Arch Biochem Biophys 253:271–281
Pabarcus MK, Casida JE (2002) Kynurenine formamidase: determination of primary structure and modeling-based prediction of tertiary structure and catalytic triad. Biochim Biophys Acta 1596:201–211
Ohshima H, Friesen M, Brouet I, Bartsch H (1990) Nitrotyrosine as a new marker for endogenous nitrosation and nitration of proteins. Food Chem Toxicol 28:647–652
Ahmed N, Ahmed U, Thornalley PJ, Hager K, Fleischer GA, Munch G (2004) Protein glycation, oxidation and nitration marker residues and free adducts of cerebrospinal fluid in Alzheimer’s disease and link to cognitive impairment. J Neurochem 92:255–263
Ivanov VT, Yatskin ON, Kalinina OA, Philippova MM, Karelin AA, Blishchenko EY (2000) Tissue-specific peptide pools. Generation and function. Pure Appl Chem 72:355–363
Greive KA, Balazs NDH, Comper WD (2001) Protein fragments in urine have been considerably underestimated by various protein assays. Clin Chem 47:1717–1719
Ceriello A, Mercuri F, Quagliaro L et al (2001) Detection of nitrotyrosine in the diabetic plasma: evidence of oxidative stress. Diabetologia 44:834–838
Soriano FG, Virag L, Jagtap P et al (2001) Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med 7:108–113
Gladwin MT, Lancaster JR, Freeman BA, Schechter AN (2003) Nitric oxide’s reactions with hemoglobin: a view through the SNO-storm. Nat Med 9:496–500
Acknowledgements
We thank the Juvenile Diabetes Research Fund (USA) and the Wellcome Trust (UK), the Eli Lilly Company and the American Diabetes Association for supporting our research. P. J. Thornalley and P. J. Beisswenger are co-senior authors.
Duality of interest P. J. Beisswenger received consulting fees from, and currently serves on the Professional Speaking Bureau for, Eli Lilly and Company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmed, N., Babaei-Jadidi, R., Howell, S.K. et al. Degradation products of proteins damaged by glycation, oxidation and nitration in clinical type 1 diabetes. Diabetologia 48, 1590–1603 (2005). https://doi.org/10.1007/s00125-005-1810-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-005-1810-7