Abstract
Summary
This 6-month randomized double-blind placebo-controlled trial shows that risedronate is well tolerated and effective in improving lumbar spine BMD and reducing loss of BMD at the hips in patients receiving high-dose prednisolone.
Introduction
Bisphosphonates have proven benefits in patients receiving chronic low-dose glucocorticoids. However, whether they are effective in preventing bone mineral density (BMD) loss during periods of high-dose glucocorticoid treatment is unclear. The objective of this paper is to study the efficacy of risedronate in preventing bone mineral density (BMD) loss in users of high-dose glucocorticoids.
Methods
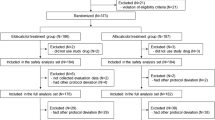
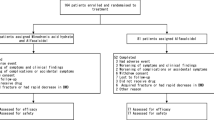
Adult patients with medical diseases treated with high-dose prednisolone (>0.5 mg/kg/day) were randomized to receive risedronate (5 mg/day) or placebo for 6 months in a double-blind manner, along with elemental calcium (1,000 mg/day). Changes in BMD were studied.
Results
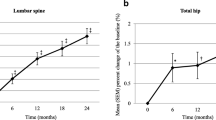
One hundred and twenty patients were recruited (82 women, age 42.8 ± 14.3 years, 63% corticosteroid-naive, 30% women postmenopausal) and 103 completed the study. Baseline clinical characteristics and BMD were similar in the risedronate and placebo groups. At 6 months, a significant gain in spinal BMD was observed in the risedronate group (+0.7 ± 0.3%; p = 0.03) but a drop was detected in the placebo group (−0.7 ± 0.4%; p = 0.12). After adjustment for baseline BMD, age, gender, body mass index and cumulative prednisolone dosages, the inter-group difference in spinal BMD remained significant (1.4%; p = 0.006). Both groups had a significant drop in hip BMD, but the magnitude was greater in the placebo arm (−0.8 ± 0.4% in risedronate versus −1.3 ± 0.5% the in placebo). No new fractures developed. Subgroup analysis of corticosteroid-naive patients yielded similar results. Upper gastrointestinal adverse events were numerically more frequent in the risedronate group.
Conclusions
Risedronate improves spinal BMD in users of high-dose glucocorticoids.


Similar content being viewed by others
References
Shaker JL, Lukert BP (2005) Osteoporosis associated with excess glucocorticoids. Endocrinol Metab Clin North Am 34:341–56, viii-ix
Angeli A, Guglielmi G, Dovio A et al (2006) High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone 39:253–259
Eastell R, Reid DM, Compston J et al (1998) A UK Consensus Group on management of glucocorticoid-induced osteoporosis: an update. J Intern Med 244:271–292
Mazziotti G, Angeli A, Bilezikian JP et al (2006) Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab 17:144–149
de Nijs RN, Jacobs JW, Bijlsma JW et al; Osteoporosis Working Group, Dutch Society for Rheumaology (2001) Prevalence of vertebral deformities and symptomatic vertebral fractures in corticosteroid treated patients with rheumatoid arthritis. Rheumatology (Oxford) 40:1375–1383
Van Staa TP, Laan RF, Barton IP et al (2003) Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 48:3224–3229
Van Staa TP, Leufkens HG, Abenhaim L et al (2000) Use of oral corticosteroids and risk of fractures. J Bone Miner Res 15:993–1000
Haugeberg G, Griffiths B, Sokoll KB et al (2004) Bone loss in patients treated with pulses of methylprednisolone is not negligible: a short term prospective observational study. Ann Rheum Dis 63:940–944
Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis (2001) Arthritis Rheum 44:1496–1503
Devogelaer JP, Goemaere S, Boonen S et al (2006) Evidence-based guidelines for the prevention and treatment of glucocorticoid-induced osteoporosis: a consensus document of the Belgian Bone Club. Osteoporos Int 17:8–19
Adachi JD, Bensen WG, Brown J et al (1997) Intermittent etidronate therapy to prevent corticosteroid-induced osteoporosis. N Engl J Med 337:382–7
Saag KG, Emkey R, Schnitzer TJ et al (1998) Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med 339:292–299
Eastell R, Devogelaer JP, Peel NF et al (2000) Prevention of bone loss with risedronate in glucocorticoid-treated rheumatoid arthritis patients. Osteoporos Int 11:331–337
Cohen S, Levy RM, Keller M et al (1999) Risedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 42:2309–2318
Reid DM, Hughes RA, Laan RF et al (2000) Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-Induced Osteoporosis Treatment Study. J Bone Miner Res 15:1006–1013
Wallach S, Cohen S, Reid DM et al (2000) Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int 67:277–285
Lems WF, Lodder MC, Lips P et al (2006) Positive effect of alendronate on bone mineral density and markers of bone turnover in patients with rheumatoid arthritis on chronic treatment with low-dose prednisone: a randomized, double-blind, placebo-controlled trial. Osteoporos Int 17:716–723
Nzeusseu Toukap A, Depresseux G, Devogelaer JP et al (2005) Oral pamidronate prevents high-dose glucocorticoid-induced lumbar spine bone loss in premenopausal connective tissue disease (mainly lupus) patients. Lupus 14:517–520
Looker AC, Wahner HW, Dunn WL et al (1998) Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int 8:468–489
Kung AW, Chan TM, Lau CS et al (1999) Osteopenia in young hypogonadal women with systemic lupus erythematosus receiving chronic steroid therapy: a randomized controlled trial comparing calcitriol and hormonal replacement therapy. Rheumatology (Oxford) 38:1239–1244
Lambrinoudaki I, Chan DT, Lau CS et al (2000) Effect of calcitriol on bone mineral density in premenopausal Chinese women taking chronic steroid therapy. A randomized, double blind, placebo controlled study. J Rheumatol 27:1759–1765
Sambrook PN, Kotowicz M, Nash P et al (2003) Prevention and treatment of glucocorticoid-induced osteoporosis: a comparison of calcitriol, vitamin D plus calcium, and alendronate plus calcium. J Bone Miner Res 18:919–924
de Nijs RN, Jacobs JW, Lems WF et al; STOP Investigators (2006) Alendronate or alfacalcidol in glucocorticoid-induced osteoporosis. N Engl J Med 355:675–684
Jardinet D, Lefèbvre C, Depresseux G et al (2000) Longitudinal analysis of bone mineral density in pre-menopausal female systemic lupus erythematosus patients: deleterious role of glucocorticoid therapy at the lumbar spine. Rheumatology (Oxford) 39:389–392
Taggart H, Bolognese MA, Lindsay R et al (2002) Upper gastrointestinal tract safety of risedronate: a pooled analysis of 9 clinical trials. Mayo Clin Proc 77:262–270
Adami S, Pavelka K, Cline GA et al (2005) Upper gastrointestinal tract safety of daily oral risedronate in patients taking NSAIDs: a randomized, double-blind, placebo-controlled trial. Mayo Clin Proc 80:1278–1285
Acknowledgement
The risedronate and placebo drug samples were provided free of charge from Sanofi-Aventis. Apart from drug samples, there was no other financial subsidy from the drug company. None of the authors have financial interests with Sanofi-Aventis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mok, C.C., Tong, K.H., To, C.H. et al. Risedronate for prevention of bone mineral density loss in patients receiving high-dose glucocorticoids: a randomized double-blind placebo-controlled trial. Osteoporos Int 19, 357–364 (2008). https://doi.org/10.1007/s00198-007-0505-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-007-0505-y