Abstract
Summary
This study evaluated whether patients treated with bisphosphonates in the US Military Health System were more compliant with treatment given monthly versus weekly. While medication compliance did improve with treatment given monthly, overall compliance with bisphosphonates was still suboptimal suggesting the need for further strategies to improve compliance with treatment for osteoporosis.
Introduction
The study objective was to evaluate the relationship between bisphosphonate dosing interval and medication compliance among new users initiating oral bisphosphonates.
Methods
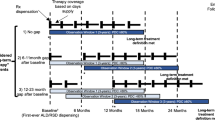
We conducted a retrospective observational cohort study of administrative claims data in the US Military Health System to examine medication compliance among 22,363 new users of oral bisphosphonates starting weekly (68%) or monthly (32%) therapy. Medication compliance during the first year of treatment was measured using two methods: (1) medication possession ratio (MPR) with compliance defined as ≥80% of days covered and (2) time to first gap of more than 30 days following initiation. Logistic regression and a proportional hazards model were used to detect differences in medication compliance between cohorts.
Results
After the first year of therapy, 57% of subjects were not compliant with bisphosphonates (MPR <80%), while 84% experienced a gap in treatment of more than 30 days. After adjustment for study covariates, the odds of a patient being compliant with treatment was 21% higher among monthly users compared to weekly users (OR 1.207, 95% confidence interval (CI) 1.119–1.257). Similarly, the risk of experiencing a 30-day gap in treatment was 6% lower among monthly users compared to weekly users (HR 0.934, 95% CI 0.905–0.964).
Conclusions
Patients receiving oral bisphosphonates on a monthly basis showed higher rates of medication compliance compared to weekly dosing in our study. However, compliance with bisphosphonates among all new users was suboptimal, suggesting the need for improved strategies to enhance compliance with oral bisphosphonates in the US Military Health System.


Similar content being viewed by others
References
US Department of Health and Human Services (2004) Bone health and osteoporosis: a report of the surgeon general. US Department of Health and Human Services, Rockville, MD. http://www.surgeongeneral.gov/library/bonehealth/. Accessed 16 Nov 2010
Randell AG, Nguyen TV, Bhalerao N, Silverman SL, Sambrook PN, Eisman JA (2000) Deterioration in quality of life following hip fracture: a prospective study. Osteoporos Int 11(5):460–466. doi:10.1007/s001980070115
Gabriel SE, Tosteson AN, Leibson CL et al (2002) Direct medical costs attributable to osteoporotic fractures. Osteoporos Int 13(4):323–330. doi:10.1007/s001980200033
Bluic D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR (2009) Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 301(5):513–521. doi:10.1001/jama.2009.50
Black DM, Cummings SR, Karpf DB et al (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 348(9041):1535–1541. doi:10.1016/S0140-6736(96)07088-2
Papapoulos SE (2001) Bisphosphonates in the management of postmenopausal osteoporosis. In: Marcus R, Feldman D, Kelsey J (eds) Osteoporosis. vol. 2, 2nd edn. Academic, San Diego, pp 631–649
Compston J (2010) Management of glucocorticoid-induced osteoporosis. Nat Rev Rheumatol 6(2):82–88
Solomon DH, Avorn J, Katz J et al (2005) Compliance with osteoporosis medications. Arch Intern Med 165(20):2414–2419
Claxton AJ, Cramer J, Pierce C (2001) A systematic review of the associations between dose regimens and medication compliance. Clin Ther 23(8):1296–1310. doi:10.1016/S0149-2918(01)80109-0
Rabenda V, Hiligsmann M, Reginster JY (2009) Poor adherence to oral bisphosphonate treatment and its consequences: a review of the evidence. Expert Opin Pharmacother 10(14):2303–2315. doi:10.1517/14656560903140533
Cramer JA, Gold DT, Silverman SL, Lewiecki EM (2007) A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int 18:1023–1031. doi:10.1007/s00198-006-0322-8
Briesacher BA, Andrade SE, Harrold LR, Fouayzi H, Yood RA (2010) Adoption of once-monthly oral bisphosphonates and the impact on adherence. Am J Med 123(3):275–280. doi:10.1016/j.amjmed.2009.05.017
Cramer JA, Silverman SL, Gold DT (2007) Methodological considerations in using claims databases to evaluate persistence with bisphosphonates for osteoporosis. Curr Med Res Opin 23(10):2369–2377. doi:10.1185/030079907X226311
Cramer JA, Roy A, Burrell A et al (2008) Medication compliance and persistence: terminology and definitions. Value Health 11(1):44–47
Peterson AM, Nau DP, Cramer JA et al (2007) A checklist for medication compliance and persistence studies using retrospective databases. Value Health 10(1):3–12
Weiss TW, Henderson SC, McHorney CA, Cramer JA (2007) Persistence across weekly and monthly bisphosphonates: analysis of US retail pharmacy prescription refills. Curr Med Res Opin 23(9):2193–2203. doi:10.1185/030079907X226069
Cooper A, Drake J, Brankin E, PERSIST Investigators (2006) Treatment persistence with once-monthly ibandronate and patient support vs. once-weekly alendronate: results from the PERSIST study. Int J Clin Pract 60(8):896–905. doi:10.1111/j.1742-1241.2006.01059.x
Papaioannou A, Kennedy CC, Dolovich L, Lau E, Adachi JD (2007) Patient adherence to osteoporosis medications: problems, consequences and management strategies. Drugs Aging 24(1):37–55
Rossini M, Bianchi G, Di Munno O et al (2006) Determinants of adherence to osteoporosis treatment in clinical practice. Osteoporos Int 17(6):914–921. doi:10.1007/s00198-006-0073-6
Sellmeyer DE (2010) Atypical fractures as a potential complication of long-term bisphosphonate therapy. JAMA 304(13):1480–1484. doi:10.1001/jama.2010.1360
Bonnick SL, Silverman S, Tanner SB et al (2009) Patient satisfaction in postmenopausal women treated with a weekly bisphosphonate transitioned to once-monthly ibandronate. J Wom Health (Larchmt) 18(7):935–943
Silverman SL, Schousboe JT, Gold DT (2011) Oral bisphosphonate compliance and persistence: a matter of choice? Osteoporos Int 22(1):21–26. doi:10.1007/s00198-010-1274-6
Binkley N, Martens MG, Silverman SL et al (2009) Improved GI tolerability with monthly ibandronate in women previously using weekly bisphosphonates. South Med J 102(5):486–492. doi:10.1097/SMJ.0b013e31819ed0dd
Blumentals WA, Harris ST, Cole RE, Huang L, Silverman SL (2009) Risk of severe gastrointestinal events in women treated with monthly ibandronate or weekly alendronate and risedronate. Ann Pharmacother 43(4):577–585. doi:10.1345/aph.1L555
Grey A, Bolland M, Wattie D, Horne A, Gamble G, Reid IR (2010) Prolonged antiresorptive activity of zolendronate: a randomized, controlled trial. J Bone Miner Res 25(10):2251–2255. doi:10.1002/jbmr.103
Bolognese MA, Civitelli R, Drezner M, Emkey R (2005) Intravenous ibandronate injections with extended dosing intervals provide at least equivalent efficacy to daily oral ibandronate: 1-year results from the DIVA study. Presented at the Sixth international symposium on osteoporosis: current status and future directions. National Osteoporosis Foundation, Washington DC, April 6. http://nof.confex.com/nof/2005/techprogram/P272.HTM. Accessed 28 Dec 2010
Siris ES, Harris ST, Rosen CJ et al (2006) Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc 81(8):1013–1022. doi:10.4065/81.8.1013
Yood RA, Emani S, Reed JI, Lewis BE, Charpentier M, Lydick E (2003) Compliance with pharmacologic therapy for osteoporosis. Osteoporos Int 14(12):965–968. doi:10.1007/s00198-003-1502-4
Acknowledgments
The authors gratefully acknowledge Deborah Garcia for administrative and technical support during the drafting of this manuscript.
Disclaimer
The thoughts and opinions expressed in this paper are those of the authors and do not necessarily represent the official policy or position of TRICARE Management Activity, the Department of Defense, or the US Government.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Devine, J., Trice, S., Finney, Z. et al. A retrospective analysis of extended-interval dosing and the impact on bisphosphonate compliance in the US Military Health System. Osteoporos Int 23, 1415–1424 (2012). https://doi.org/10.1007/s00198-011-1729-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-011-1729-4