Abstract
Summary
The efficacy and safety of weekly oral odanacatib (ODN) 50 mg for up to 8 years were assessed in postmenopausal women with low bone mineral density (BMD). Treatment with ODN for up to 8 years resulted in continued or maintained increases in BMD at multiple sites and was well tolerated.
Introduction
ODN is a selective inhibitor of cathepsin K. In a 2-year phase 2b study (3/10/25/50 mg ODN once weekly [QW] or placebo) and extensions (50 mg ODN QW or placebo), ODN treatment for 5 years progressively increased BMD and decreased bone resorption markers in postmenopausal women with low BMD (ClinicalTrials.gov NCT00112437).
Methods
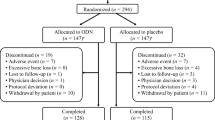
In this prespecified interim analysis at year 8 of an additional 5-year extension (years 6 to 10), patients (n = 117) received open-label ODN 50 mg QW plus weekly vitamin D3 (5600 IU) and calcium supplementation as needed. Primary end points were lumbar spine BMD and safety. Patients were grouped by ODN exposure duration.
Results
Mean (95 % confidence interval [CI]) lumbar spine BMD changes from baseline were 4.6 % (2.4, 6.7; 3-year continuous ODN exposure), 12.9 % (8.1, 17.7; 5 years), 12.8 % (10.0, 15.7; 6 years), and 14.8 % (11.0, 18.6; 8 years). Similar patterns of results were observed for BMD of trochanter, femoral neck, and total hip versus baseline. Geometric mean changes from baseline to year 8 for bone resorption markers were approximately −50 % (uNTx/Cr) and −45 % (sCTx), respectively (all groups); bone formation markers remained near baseline levels. No osteonecrosis of the jaw, delayed fracture union, or morphea-like skin reactions were reported.
Conclusions
Treatment with ODN for up to 8 years resulted in gains in BMD at multiple sites. Bone resorption markers remained reduced, with no significant change observed in bone formation markers. Treatment with ODN for up to 8 years was well tolerated.



Similar content being viewed by others
References
NIH Consensus Development Panel (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377:1276–1287
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22:465–475
Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24:23–57
Rizzoli R, Branco J, Brandi ML et al (2014) Management of osteoporosis of the oldest old. Osteoporos Int 25:2507–2529
Whitaker M, Guo J, Kehoe T, Benson G (2012) Bisphosphonates for osteoporosis—where do we go from here? N Engl J Med 366:2048–2051
Eli Lilly and Company (2012) FORTEO prescribing information. www.forteo.com. Accessed 26 Jan 2016
Gauthier JY, Chauret N, Cromlish W et al (2008) The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg Med Chem Lett 18:923–928
Cusick T, Chen CM, Pennypacker BL et al (2012) Odanacatib treatment increases hip bone mass and cortical thickness by preserving endocortical bone formation and stimulating periosteal bone formation in the ovariectomized adult rhesus monkey. J Bone Miner Res 27:524–537
Masarachia PJ, Pennypacker BL, Pickarski M et al (2012) Odanacatib reduces bone turnover and increases bone mass in the lumbar spine of skeletally mature ovariectomized rhesus monkeys. J Bone Miner Res 27:509–523
Stoch SA, Zajic S, Stone J et al (2009) Effect of the cathepsin K inhibitor odanacatib on bone resorption biomarkers in healthy postmenopausal women: two double-blind, randomized, placebo-controlled phase I studies. Clin Pharmacol Ther 86:175–182
Bone HG, McClung MR, Roux C et al (2010) Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone density. J Bone Miner Res 25:937–947
Eisman JA, Bone HG, Hosking DJ et al (2011) Odanacatib in the treatment of postmenopausal women with low bone mineral density: three-year continued therapy and resolution of effect. J Bone Miner Res 26:242–251
Langdahl B, Binkley N, Bone H et al (2012) Odanacatib in the treatment of postmenopausal women with low bone mineral density: five years of continued therapy in a phase 2 study. J Bone Miner Res 27:2251–2258
Sassi ML, Eriksen H, Risteli L et al (2000) Immunochemical characterization of assay for carboxyterminal telopeptide of human type I collagen: loss of antigenicity by treatment with cathepsin K. Bone 26:367–373
Rünger TM, Adami S, Benhamou CL et al (2012) Morphea-like skin reactions in patients treated with the cathepsin K inhibitor balicatib. J Am Acad Dermatol 66:e89–e96
Peroni A, Zini A, Braga V, Colato C, Adami S, Girolomoni G (2008) Drug-induced morphea: report of a case induced by balicatib and review of the literature. J Am Acad Dermatol 59:125–129
Kawalilak CE, Johnston JD, Olszynski WP, Kontulainen SA (2014) Characterizing microarchitectural changes at the distal radius and tibia in postmenopausal women using HR-pQCT. Osteoporos Int 25:2057–2066
Seeman E, Delmas PD, Hanley DA et al (2010) Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res 25:1886–1894
McClung MR, Lewiecki EM, Cohen SB et al (2006) Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 354:821–831
Cheung AM, Majumbdar S, Brixen K et al (2014) Effects of odanacatib on the radius and tibia of postmenopausal women: improvements in bone geometry, microarchitecture, and estimated bone strength. J Bone Miner Res 29:1786–1794
Pennypacker BL, Gilberto D, Gatto NT et al (2016) Odanacatib increases mineralized callus during fracture healing in a rabbit ulnar osteotomy model. J Orthop Res 34:72–80
Pennypacker BL, Chen CM, Zheng H et al (2014) Inhibition of cathepsin K increases modeling-based bone formation, and improves cortical dimension and strength in adult ovariectomized monkeys. J Bone Miner Res 29:1847–1858
Ominsky MS, Libanati C, Niu QT et al (2015) Sustained modeling-based bone formation during adulthood in cynomolgus monkeys may contribute to continuous BMD gains with denosumab. J Bone Miner Res 30:1280–1289
Brixen K, Chapurlat R, Cheung AM et al (2013) Bone density, turnover, and estimated strength in postmenopausal women treated with odanacatib: a randomized trial. J Clin Endocrinol Metab 98:571–580
Halleen JM, Tiitinen SL, Ylipahkala H, Fagerlund KM, Vaananen HK (2006) Tartrate-resistant acid phosphatase 5b (TRACP 5b) as a marker of bone resorption. Clin Lab 52:499–509
Pennypacker B, Shea M, Liu Q et al (2009) Bone density, strength, and formation in adult cathepsin K (−/−) mice. Bone 44:199–207
Baron R, Ferrari S, Russell RG (2011) Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 48:677–692
Jensen PR, Andersen TL, Pennypacker BL, le Duong T, Delaissé JM (2014) The bone resorption inhibitors odanacatib and alendronate affect post-osteoclastic events differently in ovariectomized rabbits. Calcif Tissue Int 94:212–222
Sims NA, Ng KW (2014) Implications of osteoblast-osteoclast interactions in the management of osteoporosis by antiresorptive agents denosumab and odanacatib. Curr Osteoporos Rep 12:98–106
McClung M, Harris ST, Miller PD et al (2013) Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med 126:13–20
Sutton EE, Riche DM (2012) Denosumab, a RANK ligand inhibitor, for postmenopausal women with osteoporosis. Ann Pharmacother 46:1000–1009
McClung MR, Langdahl B, Papapoulos S et al (2015) Odanacatib anti-fracture efficacy and safety in postmenopausal women with osteoporosis: results from the phase III Long-Term Odanacatib Fracture Trial (LOFT). IBMS BoneKEy 13:Article number:677. Abstract OC4.4
Acknowledgments
This study was sponsored by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ. Medical writing support was provided by Annette Smith, PhD, of Complete Medical Communications, funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ. Authors’ roles: study design CD, NV, and AL; study conduct CD, AL, and DG; data collection JH, IR, JARP, CD, RK, and DG; data analysis RR, PDM, RK, NV, AL, and DG; data interpretation RR, JH, PDM, CD, RK, NV, AL, and DG; statistical expertise NV and RK; revising manuscript content RR, C-LB, JH, PDM, IR, JARP, CD, RK, NV, AL, and DG; approved final version RR, C-LB, JH, PDM, IR, JARP, CD, RK, NV, AL, and DG; and RK, NV, AL, and DG take responsibility for the integrity of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
R. Rizzoli has received fees for board membership from Amgen, Danone, Merck, Servier, and Takeda and lecture fees from Amgen, Danone, GSK, Merck, and Servier. C.-L. Benhamou has received fees for board membership from Rottapharm, Pierre Fabre, Amgen, Novartis, and MSD and grants from Amgen and Servier. J. Halse has received a grant and travel expenses to an investigator meeting from MSD Norway AS for this study and consultancy fees from MSD Norway AS, Eli Lilly Norway AS, grants from Amgen AB Norway, and lecture fees from GSK Norway AS and Amgen AB Norway. P. D. Miller has participated in scientific advisory boards for Alexion, Amgen, AgNovos, Lilly, Merck, Radius Pharma, and Roche and in speaker bureaus for Alexion Pharmaceuticals, Amgen, and Radius Health. He has received research grants from Alexion, Amgen, Boehringer Ingelheim, Immunodiagnostics, Lilly, Merck, Merck Serono, NBHA, Novartis, Novo Nordisk, Radius Pharma, Roche Diagnostics, and Takeda. I.R. Reid has received fees from Merck for consultancy, lectures, and board membership, and his institution has received a grant from Merck for this study. J. A. Rodríguez Portales has received travel expenses to attend an investigator meeting, and his institution has received grants from Merck for this and other studies. C. DaSilva, N. Verbruggen, and D. Gurner are employees of and hold stock/stock options in Merck & Co., Inc. R. Kroon was an employee of MSD at the time of the study. A.T. Leung was an employee of Merck & Co., Inc. at the time of the study and currently holds stocks of the company.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material
(PDF 975 kb)
Rights and permissions
About this article
Cite this article
Rizzoli, R., Benhamou, CL., Halse, J. et al. Continuous treatment with odanacatib for up to 8 years in postmenopausal women with low bone mineral density: a phase 2 study. Osteoporos Int 27, 2099–2107 (2016). https://doi.org/10.1007/s00198-016-3503-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3503-0