Abstract
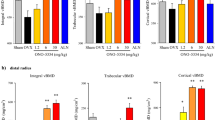
The cysteine protease cathepsin K is involved in osteoclast-mediated bone resorption. We evaluated the effect of daily oral dosing of an inhibitor of human cathepsin K (SB-462795 [relacatib]) for 9 months on bone turnover, mass, and architecture in estrogen-deficient cynomolgus monkeys. Ovariectomized animals were treated orally with relacatib at 1, 3, or 10 mg/kg/day, or oral vehicle plus alendronate at 0.05 mg/kg by IV injection once every 2 weeks. The control groups, ovariectomized and sham-ovariectomized animals, received vehicle (all groups n = 20 animals). Samples for biomarker analysis were collected at various times, bone mass changes were evaluated at 6 and 9 months of treatment, and histomorphometric analysis was performed at 9 months. Relacatib significantly reduced urinary N-telopeptide excretion within 1 week of treatment at all dose levels, an effect that was maintained at the highest dose level. At some time points bone formation markers were elevated at the lowest dose of relacatib. Animals treated with relacatib had dose-dependent preservation of areal bone mineral density reaching statistical significance in distal femur. In femur neck there was significant preservation of total volumetric BMD (vBMD) by relacatib. By histomorphometry, relacatib reduced indices of bone resorption and formation at cancellous sites as did alendronate. In cortical bone, osteonal bone formation rate was reduced by alendronate but preserved at low and medium doses of relacatib. Thus, relacatib preserved cortical and cancellous bone mass in ovariectomized monkeys.


Similar content being viewed by others
References
National Osteoporosis Foundation (2002) America’s bone health: the state of osteoporosis and low bone mass in our nation. National Osteoporosis Foundation, Washington, DC
Reginster JY, Burlet N (2006) Osteoporosis: a still increasing prevalence. Bone 38(2 Suppl 1):4–9
Yasuda Y, Kaleta J, Bromme D (2005) The role of cathepsins in osteoporosis and arthritis: rationale for the design of new therapeutics. Adv Drug Deliv Rev 57(7):973–993
Grey A, Reid IR (2005) Emerging and potential therapies for osteoporosis. Expert Opin Investig Drugs 14(3):265–278
Sambrook PN, Rodriguez JP, Wasnich RD, Luckey MM, Kaur A, Meng L, Lombardi A (2004) Alendronate in the prevention of osteoporosis: 7-year follow-up. Osteoporos Int 15(6):483–488
Nguyen ND, Eisman JA, Nguyen TV (2006) Anti-hip fracture efficacy of bisphosphonates: a Bayesian analysis of clinical trials. J Bone Miner Res 21(1):340–349
Schneider JP (2006) Should bisphosphonates be continued indefinitely? An unusual fracture in a healthy woman on long-term alendronate. Geriatrics 61(1):31–33
Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY (2005) Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab 90(3):1294–1301
Porras AG, Holland SD, Gertz BJ (1999) Pharmacokinetics of alendronate. Clin Pharmacokinet 36(5):315–328
Marshall JK (2002) The gastrointestinal tolerability and safety of oral bisphosphonates. Exp Opin Drug Safety 1(1):71–78
Reginster JY, Felsenberg D, Cooper C, Stakkestad JA, Miller PD, Kendler DL, Adami S, McClung MR, Bolognese MA, Civitelli R, Dumont E, Bonvoisin B, Recker RR, Delmas PD (2006) A new concept for bisphosphonate therapy: a rationale for the development of monthly oral dosing of ibandronate. Osteoporos Int 17(2):159–166
Reginster JY (2006) Adherence and persistence: impact on outcomes and health care resources. Bone 38(2 Suppl 2):18–21
Bilezikian JP (2005) Anabolic therapy for osteoporosis. Int J Fertil Womens Med 50(2):53–60
Rubin MR, Bilezikian JP (2005) Parathyroid hormone as an anabolic skeletal therapy. Drugs 65(17):2481–2498
Poole KE, Reeve J (2005) Parathyroid hormone—a bone anabolic and catabolic agent. Curr Opin Pharmacol 5(6):612–617
Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK (2005) Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev 26(5):688–703
Bilezikian JP, Rubin MR, Finkelstein JS (2005) Parathyroid hormone as an anabolic therapy for women and men. J Endocrinol Invest 28(8 Suppl):41–49
Lecart MP, Bruyere O, Reginster JY (2004) Combination/sequential therapy in osteoporosis. Curr Osteoporos Rep 2(4):123–130
Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, Lang TF, McGowan JA, Rosen CJ (2005) One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med 353(6):555–565
Takahashi N, Udagawa M, Takami M, Suda T (2002) Cells of bone: osteoclast generation. In: Bilezikian JP, Raisz LG, Rodan GA (eds) Principles of bone biology, 2nd edn. Academic Press, San Diego, pp 109–126
Wang D, Miller SC, Kopeckova P, Kopecek J (2005) Bone-targeting macromolecular therapeutics. Adv Drug Deliv Rev 57(7):1049–1076
Väänänen K, Zhao H (2002) Osteoclast function: biology and mechanism. In: Bilezikian JP, Raisz LG, Rodan GA (eds) Principles of bone biology, 2nd edn. Academic Press, San Diego, pp 127–139
Drake FH, Dodds RA, James IE, Connor JR, Debouck C, Richardson S, Lee-Rykaczewski E, Coleman L, Rieman D, Barthlow R, Hastings G, Gowen M (1996) Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J Biol Chem 271(21):12511–12516
Gowen M, Lazner F, Dodds R, Kapadia R, Feild J, Tavaria M, Bertoncello I, Drake F, Zavarselk S, Tellis I, Hertzog P, Debouck C, Kola I (1999) Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J Bone Miner Res 14(10):1654–1663
Saftig P, Hunziker E, Wehmeyer O, Jones S, Boyde A, Rommerskirch W, Moritz JD, Schu P, von Figura K (1998) Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci USA 95(23):13453–13458
Marquis RW, Ru Y, Yamashita DS, Oh HJ, Yen J, Thompson SK, Carr TJ, Levy MA, Tomaszek TA, Ijames CF, Smith WW, Zhao B, Janson CA, Abdel-Meguid SS, D’Alessio KJ, McQueney MS, Veber DF (1999) Potent dipeptidylketone inhibitors of the cysteine protease cathepsin K. Bioorg Med Chem 7(4):581–588
Votta BJ, Levy MA, Badger A, Bradbeer J, Dodds RA, James IE, Thompson S, Bossard MJ, Carr T, Connor JR, Tomaszek TA, Szewczuk L, Drake FH, Veber DF, Gowen M (1997) Peptide aldehyde inhibitors of cathepsin K inhibit bone resorption both in vitro and in vivo. J Bone Miner Res 12(9):1396–1406
Yamashita DS, Marquis RW, Xie R, Nidamarthy SD, Oh HJ, Jeong JU, Erhard KF, Ward KW, Roethke TJ, Smith BR, Cheng HY, Geng X, Lin F, Offen PH, Wang B, Nevins N, Head MS, Haltiwanger RC, Narducci Sarjeant AA, Liable-Sands LM, Zhao B, Smith WW, Janson CA, Gao E, Tomaszek T, McQueney M, James IE, Gress CJ, Zembryki DL, Lark MW, Veber DF (2006) Structure activity relationships of 5-, 6-, and 7-methyl-substituted azepan-3-one cathepsin K inhibitors. J Med Chem 49(5):1597–1612
Kumar S, Dare L, Vasko-Moser JA, James IE, Blake SM, Rickard DJ, Hwang SM, Tomaszek T, Yamashita DS, Marquis RW, Oh H, Jeong JU, Veber DF, Gowen M, Lark MW, Stroup G (2006) A highly potent inhibitor of cathepsin K (relacatib) reduces biomarkers of bone resorption both in vitro and in an acute model of elevated bone turnover in vivo in monkeys. Bone 40(1):122–131
Kumar S, Rehm S, Boyce R, Birmingham J, Stroup G, Jerome C, Wier P (2005) Treatment of young male monkeys for 12 months with a highly potent inhibitor of Cathepsin K inhibits bone resorption and increases bone mineral density and strength. J Bone Miner Res 20(Suppl 1):S81
Jerome CP (1998) Primate models of osteoporosis. Lab Anim Sci 48(6):618–622
Rosen CJ, Hochberg MC, Bonnick SL, McClung M, Miller P, Broy S, Kagan R, Chen E, Petruschke RA, Thompson DE, de Papp AE (2005) Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res 20(1):141–151
Bayne K (1996) Revised guide for the care and use of laboratory animals available. American Physiological Society. Physiologist 39(4):199, 208–211
Thompson DD, Seedor JG, Quartuccio H, Solomon H, Fioravanti C, Davidson J, Klein H, Jackson R, Clair J, Frankenfield D et al (1992) The bisphosphonate, alendronate, prevents bone loss in ovariectomized baboons. J Bone Miner Res 7(8):951–960
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2(6):595–610
Jerome CP, Power RA, Obasanjo IO, Register TC, Guidry M, Carlson CS, Weaver DS (1997) The androgenic anabolic steroid nandrolone decanoate prevents osteopenia and inhibits bone turnover in ovariectomized cynomolgus monkeys. Bone 20(4):355–364
Jerome CP, Burr DB, Van Bibber T, Hock JM, Brommage R (2001) Treatment with human parathyroid hormone (1–34) for 18 months increases cancellous bone volume and improves trabecular architecture in ovariectomized cynomolgus monkeys (Macaca fascicularis). Bone 28(2):150–159
Parfitt A, Foldes J (1991) The ambiguity of interstitial bone thickness: a new approach to the mechanism of trabecular thinning. Bone 12:119–122
Deaton DN, Kumar S (2004) Cathepsin K inhibitors: their potential as anti-osteoporosis agents. Prog Med Chem 42:245–375
Jerome CP, Peterson PE (2001) Nonhuman primate models in skeletal research. Bone 29(1):1–6
Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD (1996) Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res 11(3):337–349
Stroup GB, Hoffman SJ, Vasko-Moser JA, Lechowska BA, Jenkins EL, Dare LC, Gowen M (2001) Changes in bone turnover following gonadotropin-releasing hormone (GnRH) agonist administration and estrogen treatment in cynomolgus monkeys: a short-term model for evaluation of antiresorptive therapy. Bone 28(5):532–537
Bonnick SL, Shulman L (2006) Monitoring osteoporosis therapy: bone mineral density, bone turnover markers, or both? Am J Med 119(4 Suppl 1):S25–S31
Chesnut CH 3rd, Rosen CJ (2001) Reconsidering the effects of antiresorptive therapies in reducing osteoporotic fracture. J Bone Miner Res 16(12):2163–2172
McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, Donley DW, Dalsky GP, Eriksen EF (2005) Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 165(15):1762–1768
Jerome CP, Carlson CS, Register TC, Bain FT, Jayo MJ, Weaver DS, Adams MR (1994) Bone functional changes in intact, ovariectomized, and ovariectomized, hormone-supplemented adult cynomolgus monkeys (Macaca fascicularis) evaluated by serum markers and dynamic histomorphometry. J Bone Miner Res 9(4):527–540
Recker R, Masarachia P, Santora A, Howard T, Chavassieux P, Arlot M, Rodan G, Wehren L, Kimmel D (2005) Trabecular bone microarchitecture after alendronate treatment of osteoporotic women. Curr Med Res Opin 21(2):185–194
Jerome CP, Johnson CS, Vafai HT, Kaplan KC, Bailey J, Capwell B, Fraser F, Hansen L, Ramsay H, Shadoan M, Lees CJ, Thomsen JS, Mosekilde L (1999) Effect of treatment for 6 months with human parathyroid hormone (1–34) peptide in ovariectomized cynomolgus monkeys (Macaca fascicularis). Bone 25(3):301–309
Rizzoli R (2006) Long-term outcome of weekly bisphosphonates. Clin Orthop Relat Res 443:61–65
Ravn P (2002) Bisphosphonates for prevention of postmenopausal osteoporosis. Dan Med Bull 49(1):1–18
Deaton DN, Tavares FX (2005) Design of cathepsin k inhibitors for osteoporosis. Curr Top Med Chem 5(16):1639–1675
Jerome C, Missbach M, Gamse R (2005) AAE581, a novel cathepsin K inhibitor, protects against ovariectomy-induced bone loss in non-human primates, in part by stimulation of periosteal bone formation. J Bone Miner Res 20(Suppl 1):S46
Stroup G, Dare L, Vasko-Moser J, Hoffman S, Kumar S (2006) Repeat daily dosing with a highly potent inhibitor of cathepsin K results in significant, transient elevation of plasma PTH in cynomolgus monkeys. J Bone Miner Res 21(Suppl 1):S160
Itabashi A, Kurata N, Sugita Y, Kawai Y (2006) Balicatib, a novel cathepsin K-inhibitor, increases serum intact PTH beyond diurnal patterns following 14-daily administration in Japanese postmenopausal women. J Bone Miner Res 21(Suppl 1):S24
Acknowledgments
The authors would like to thank Pam Peterson for her tremendous assistance in the production of this manuscript. We would also like to thank Dennis Yamashita and William Clark for compound synthesis and Yanli Deng for pharmacokinetic studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stroup, G.B., Kumar, S. & Jerome, C.P. Treatment with a Potent Cathepsin K Inhibitor Preserves Cortical and Trabecular Bone Mass in Ovariectomized Monkeys. Calcif Tissue Int 85, 344–355 (2009). https://doi.org/10.1007/s00223-009-9279-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-009-9279-x