Abstract
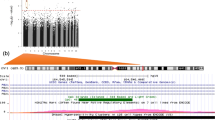
Our previous work has shown associations between childhood adiposity and perinatal methylation status of several genes in umbilical cord tissue, including endothelial nitric oxide synthase (eNOS). There is increasing evidence that eNOS is important in bone metabolism; we therefore related the methylation status of the eNOS gene promoter in stored umbilical cord to childhood bone size and density in a group of 9-year-old children. We used Sequenom MassARRAY to assess the methylation status of two CpGs in the eNOS promoter, identified from our previous study, in stored umbilical cords of 66 children who formed part of a Southampton birth cohort and who had measurements of bone size and density at age 9 years (Lunar DPXL DXA instrument). Percentage methylation varied greatly between subjects. For one of the two CpGs, eNOS chr7:150315553 + , after taking account of age and sex, there were strong positive associations between methylation status and the child’s whole-body bone area (r = 0.28, P = 0.02), bone mineral content (r = 0.34, P = 0.005), and areal bone mineral density (r = 0.34, P = 0.005) at age 9 years. These associations were independent of previously documented maternal determinants of offspring bone mass. Our findings suggest an association between methylation status at birth of a specific CpG within the eNOS promoter and bone mineral content in childhood. This supports a role for eNOS in bone growth and metabolism and implies that its contribution may at least in part occur during early skeletal development.

Similar content being viewed by others
References
Harvey N, Dennison E, Cooper C (2010) Osteoporosis: impact on health and economics. Nat Rev Rheumatol 6:99–105
Harvey N, Cooper C (2004) The developmental origins of osteoporotic fracture. J Br Menopause Soc 10:14–15 29
Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, Hsu YH, Richards JB et al (2009) Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet 41:1199–1206
Richards JB, Kavvoura FK, Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV et al (2009) Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med 151:528–537
Lanham SA, Roberts C, Perry MJ, Cooper C, Oreffo RO (2008) Intrauterine programming of bone. Part 2: alteration of skeletal structure. Osteoporos Int 19:157–167
Lanham SA, Roberts C, Cooper C, Oreffo RO (2008) Intrauterine programming of bone. Part 1: alteration of the osteogenic environment. Osteoporos Int 19:147–156
Lillycrop KA, Slater-Jefferies JL, Hanson MA, Godfrey KM, Jackson AA, Burdge GC (2007) Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br J Nutr 97:1064–1073
Lillycrop KA, Phillips ES, Torrens C, Hanson MA, Jackson AA, Burdge GC (2008) Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPAR alpha promoter of the offspring. Br J Nutr 100:278–282
Gluckman PD, Hanson MA, Cooper C, Thornburg KL (2008) Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359:61–73
Godfrey KM, Sheppard A, Gluckman PD, Lillycrop KA, Burdge GC, McLean C et al (2011) Epigenetic gene promoter methylation at birth is associated with child’s later adiposity. Diabetes 60:1528–1534
Zaman G, Pitsillides AA, Rawlinson SC, Suswillo RF, Mosley JR, Cheng MZ et al (1999) Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J Bone Miner Res 14:1123–1131
Sabanai K, Tsutsui M, Sakai A, Hirasawa H, Tanaka S, Nakamura E et al (2008) Genetic disruption of all NO synthase isoforms enhances BMD and bone turnover in mice in vivo: involvement of the renin—angiotensin system. J Bone Miner Res 23:633–643
Nilforoushan D, Gramoun A, Glogauer M, Manolson MF (2009) Nitric oxide enhances osteoclastogenesis possibly by mediating cell fusion. Nitric Oxide 21:27–36
Jamal SA, Browner WS, Bauer DC, Cummings SR (1998) Intermittent use of nitrates increases bone mineral density: the study of osteoporotic fractures. J Bone Miner Res 13:1755–1759
Godfrey K, Robinson S, Barker DJ, Osmond C, Cox V (1996) Maternal nutrition in early and late pregnancy in relation to placental and fetal growth. BMJ 312:410–414
Gale CR, Javaid MK, Robinson SM, Law CM, Godfrey KM, Cooper C (2007) Maternal size in pregnancy and body composition in children. J Clin Endocrinol Metab 92:3904–3911
Gale CR, Jiang B, Robinson SM, Godfrey KM, Law CM, Martyn CN (2006) Maternal diet during pregnancy and carotid intima-media thickness in children. Arterioscler Thromb Vasc Biol 26:1877–1882
Ehrich M, Nelson MR, Stanssens P, Zabeau M, Liloglou T, Xinarianos G et al (2005) Quantitative high-throughput analysis of DNA methylation patterns by base-specific cleavage and mass spectrometry. Proc Natl Acad Sci USA 102:15785–15790
Li LC, Dahiya R (2002) MethPrimer: designing primers for methylation PCRs. Bioinformatics 18:1427–1431
Godfrey K, Walker-Bone K, Robinson S, Taylor P, Shore S, Wheeler T et al (2001) Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J Bone Miner Res 16:1694–1703
Harvey NC, Javaid MK, Arden NK, Poole JR, Crozier SR, Robinson SM et al (2010) Maternal predictors of neonatal bone size and geometry: the Southampton Women’s Survey. J Dev Origins Health Dis 1:35–41
Talens RP, Boomsma DI, Tobi EW, Kremer D, Jukema JW, Willemsen G et al (2010) Variation, patterns, and temporal stability of DNA methylation: considerations for epigenetic epidemiology. FASEB J 24:3135–3144
Kranz AL, Eils R, Konig R (2011) Enhancers regulate progression of development in mammalian cells. Nucleic Acids Res 39:8689–8702
Park JH, Stoffers DA, Nicholls RD, Simmons RA (2008) Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest 118:2316–2324
Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ (2006) Maternal care associated with methylation of the estrogen receptor-alpha 1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology 147:2909–2915
Brunton JA, Weiler HA, Atkinson SA (1997) Improvement in the accuracy of dual energy X-ray absorptiometry for whole body and regional analysis of body composition: validation using piglets and methodologic considerations in infants. Pediatr Res 41:590–596
Chrusciel M, Andronowska A, Postek A (2009) Expression patterns of endothelial and inducible nitric oxide isoforms in the porcine umbilical cord. Reprod Domest Anim 44:621–630
Hracsko Z, Hermesz E, Ferencz A, Orvos H, Novak Z, Pal A et al (2009) Endothelial nitric oxide synthase is up-regulated in the umbilical cord in pregnancies complicated with intrauterine growth retardation. In Vivo 23:727–732
Zhang MX, Zhang C, Shen YH, Wang J, Li XN, Chen L et al (2008) Effect of 27nt small RNA on endothelial nitric-oxide synthase expression. Mol Biol Cell 19:3997–4005
Fish JE, Matouk CC, Rachlis A, Lin S, Tai SC, D’Abreo C et al (2005) The expression of endothelial nitric-oxide synthase is controlled by a cell-specific histone code. J Biol Chem 280:24824–24838
Chan Y, Fish JE, D’Abreo C, Lin S, Robb GB, Teichert AM et al (2004) The cell-specific expression of endothelial nitric-oxide synthase: a role for DNA methylation. J Biol Chem 279:35087–35100
Davis TR, Wood MB (1992) Endothelial control of long bone vascular resistance. J Orthop Res 10:344–349
Yashiro Y, Ohhashi T (1997) Flow- and agonist-mediated nitric oxide- and prostaglandin-dependent dilation in spinal arteries. Am J Physiol Heart Circ Physiol 273:H2217–H2223
Coessens BC, Miller VM, Wood MB (1996) Endothelin induces vasoconstriction in the bone vasculature in vitro: an effect mediated by a single receptor population. J Orthop Res 14:611–617
Marsh N, Marsh A (2000) A short history of nitroglycerine and nitric oxide in pharmacology and physiology. Clin Exp Pharmacol Physiol 27:313–319
Burdan F, Szumilo J, Korobowicz A, Farooquee R, Patel S, Patel A et al (2009) Morphology and physiology of the epiphyseal growth plate. Folia Histochem Cytobiol 47:5–16
Teixeira CC, Ischiropoulos H, Leboy PS, Adams SL, Shapiro IM (2005) Nitric oxide–nitric oxide synthase regulates key maturational events during chondrocyte terminal differentiation. Bone 37:37–45
Fish JE, Marsden PA (2006) Endothelial nitric oxide synthase: insight into cell-specific gene regulation in the vascular endothelium. Cell Mol Life Sci 63:144–162
Prisby RD, Ramsey MW, Behnke BJ, Dominguez JM, Donato AJ, Allen MR et al (2007) Aging reduces skeletal blood flow, endothelium-dependent vasodilation, and NO bioavailability in rats. J Bone Miner Res 22:1280–1288
Marshall D, Johnell O, Wedel H (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312:1254–1259
Acknowledgement
We thank the mothers and their children who gave us their time, and a team of dedicated research nurses and ancillary staff for their assistance. This work was supported by grants from the Medical Research Council; British Heart Foundation; Arthritis Research Campaign; National Osteoporosis Society; International Osteoporosis Foundation; Cohen Trust; Southampton NIHR Biomedical Research Unit in Nutrition, Diet and Lifestyle; and NIHR Musculoskeletal Biomedical Research Unit, University of Oxford. Participants were drawn from a cohort study funded by the Medical Research Council and the Dunhill Medical Trust. We thank Ms. G. Strange for helping to prepare the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nicholas C. Harvey and Karen A. Lillycrop are joint first author.
The authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Harvey, N.C., Lillycrop, K.A., Garratt, E. et al. Evaluation of Methylation Status of the eNOS Promoter at Birth in Relation to Childhood Bone Mineral Content. Calcif Tissue Int 90, 120–127 (2012). https://doi.org/10.1007/s00223-011-9554-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-011-9554-5