Abstract
Background
Objective quantification is critical for assessment of functional sonography in inflammatory arthritis. To create a microbubble contrast-enhanced image of vessels that lie below the resolution of a standard US system, a technique is required that detects preferentially the contrast agent echo, rejecting that from background tissue: harmonic imaging.
Objectives
To investigate the ability of contrast-enhanced triggered harmonic sonography (CETHS) to evaluate periarticular hemodynamic changes over the course of experimental arthritis and to discriminate presence and absence of arthritis based on measurement values obtained at specific time-points.
Materials and methods
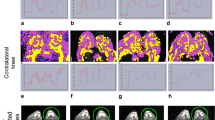
Arthritis was induced in rabbits knees by intra-articular injection of serum bovine albumin, which acted as an antigen. A total of 11 rabbits (8 with unilateral arthritis and 3 control animals) were imaged at 0, 1, 7, 14, 21 and 28 days of antigen-induced arthritis and euthanized at 28 days. A continuous infusion protocol was performed (triggering times 30.0, 20.0, 10.0, 5.0, 2.0, 1.0, and 0.5 s). Hemodynamic indices of synovial microvasculature (vascular volume, mean velocity and flow rate) were obtained and compared with clinical, laboratory, and histological surrogate markers.
Results
Although interval CETHS changes were noted for flow rate (P=0.007) and vascular volume (P=0.003) ratios in albumin-injected knees, no significant differences in ratios were identified over time between albumin-injected and non-injected knees for flow rate (P=0.52), vascular volume (P=0.23) and mean velocity (P=0.19). Flow rate most accurately differentiated between presence and absence of arthritis according to clinical measurements in early (day 1) arthritis, and mean velocity in mid-term arthritis (day 14; both P=0.02).
Conclusion
Although the measurement properties of CETHS indices were poor in the evaluation of hemodynamic differences over time in albumin-injected knees compared with non-injected knees, they enabled discrimination between presence and absence of arthritis at specific time-points in different stages.









Similar content being viewed by others
References
Edmonds SE, Blake DR, Morris CJ, et al (1993) An imaginative approach to synovitis – the role of hypoxic reperfusion damage in arthritis. J Rheumatol Suppl 37:26–31
Burns PN, Wilson SR, Muradali D, et al (1996) Microbubble destruction is the origin of harmonic signals from FS069. Radiology 201:158
Burns PN, Powers JE, Fritzsch T (1992) Harmonic imaging: a new imaging and Doppler method for contrast enhanced ultrasound. Radiology 185:142
Wilson SR, Burns PN, Muradali D, et al (2000) Harmonic hepatic US with microbubble contrast agent: initial experience showing improved characterization of hemangioma, hepatocellular carcinoma, and metastasis. Radiology 215:153–161
Burns PN, Wilson SR, Simpson DH (2000) Pulse inversion imaging of liver blood flow: improved method for characterizing focal masses with microbubble contrast. Invest Radiol 35:58–71
Harvey CJ, Blomley MJ, Eckersley RJ, et al (2000) Hepatic malignancies: improved detection with pulse-inversion US in late phase of enhancement with SH U 508A – early experience. Radiology 216:903–908
Imrie RC (1976) Animal models of arthritis. Lab Anim Sci 26:345–351
Padhani AR (2002) Dynamic contrast-enhanced MRI in clinical oncology: current status and future directions. J Magn Reson Imaging 16:407–422
Guyatt GH, Feeny DH, Patrick DL (1993) Measuring health-related quality of life. Ann Intern Med 118:622–629
Cooke TDV (1988) Antigen-induced arthritis, polyarthritis, and tenosynovitis. In: Greenwald RA, Diamond HS (eds) CRC handbook of animal models for rheumatoid diseases. CRC Press, Boca Raton, pp 53–79
Demsar F, Van Dijke CF, Kirk BA, et al (1996) Mapping abnormal synovial vascular permeability in temporomandibular joint arthritis in the rabbit using MRI. Br J Rheumatol 35 [Suppl 3]:23–25
Strouse PJ, Londy F, DiPietro MA, et al (1999) MRI evaluation of infectious and non-infectious synovitis: preliminary studies in a rabbit model. Pediatr Radiol 29:367–371
Strouse PJ, DiPietro MA, Teo EL, et al (1999) Power Doppler evaluation of joint effusions: investigation in a rabbit model. Pediatr Radiol 29:617–623
Ferrara KW, Merritt CR, Burns PN, et al (2000) Evaluation of tumor angiogenesis with US: imaging, Doppler, and contrast agents. Acad Radiol 7:824–839
Kaul S (1997) Myocardial contrast echocardiography: 15 years of research and development. Circulation 96:3745–3760
Wei K, Jayaweera AR, Firoozan S, et al (1998) Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 97:473–483
Kane D, Roth J, Frosch M, et al (2003) Increased perivascular synovial membrane expression of myeloid-related proteins in psoriatic arthritis. Arthritis Rheum 48:1676–1685
Roth J, Teigelkamp S, Wilke M, et al (1992) Complex pattern of the myelo-monocytic differentiation antigens MRP8 and MRP14 during chronic airway inflammation. Immunobiology 186:304–314
Koizumi F, Matsuno H, Wakaki K, et al (1999) Synovitis in rheumatoid arthritis: scoring of characteristic histopathological features. Pathol Int 49:298–304
Oehler S, Neureiter D, Meyer-Scholten C, et al (2002) Subtyping of osteoarthritic synoviopathy. Clin Exp Rheumatol 20:633–640
Altman DG, Bland JM (1994) Diagnostic tests 3: receiver operator characteristic plots. BMJ 309:188
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Kono Y, Mattery RF, Summers H, et al (1999) Visualization of tumor vessels with ultrasound contrast and the potential for qualitative analysis of relative tumor blood flow and fractional blood volume. Proceedings of the Bioengineering Consortium Conference, National Institutes of Health, Bethesda
Doria AS, Noseworthy M, Oakden W, et al (2006) Dynamic contrast-enhanced MRI quantification of synovium microcirculation in antigen-induced arthritis. AJR 186:1165–1171
Burns P, Wilson S (2003) Assessing antivascular therapy for hepatocellular carcinoma using US contrast agents: a baseline reproducibility study. Radiology Suppl, p 529
Johnson RG, Herbert MA, Wright S, et al (1983) The response of articular cartilage to the in vivo replacement of synovial fluid with saline. Clin Orthop Relat Res 174:285–292
Jain RK (1988) Determinants of tumor blood flow: a review. Cancer Res 48:2641–2658
Simkin PA (1979) Synovial permeability in rheumatoid arthritis. Arthritis Rheum 22:689–696
Levick JR (1990) Hypoxia and acidosis in chronic inflammatory arthritis; relation to vascular supply and dynamic effusion pressure. J Rheumatol 17:579–582
Adler DD, Carson PL, Rubin JM, et al (1990) Doppler ultrasound color flow imaging in the study of breast cancer: preliminary findings. Ultrasound Med Biol 16:553–559
Birdwell RL, Ikeda DM, Jeffrey SS, et al (1997) Preliminary experience with power Doppler imaging of solid breast masses. AJR 169:703–707
Carson PL, Moskalik AP, Govil A, et al (1997) The 3D and 2D color flow display of breast masses. Ultrasound Med Biol 23:837–849
Lassau N, Paturel-Asselin C, Guinebretiere JM, et al (1999) New hemodynamic approach to angiogenesis: color and pulsed Doppler ultrasonography. Invest Radiol 34:194–198
Lovell DJ, Giannini EH, Reiff A, et al (2000) Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric rheumatology collaborative study group. N Engl J Med 342:763–769
Acknowledgements
The authors thank Marianne Rogers for preparation of histological slides of synovium, Niels Celeghin for analysis of data, and Sheri Holmberg and Jeff Powers, PhD, for help with standardization of technical parameters at the initial phase of the study.
Definity contrast agent was funded by Bristol-Myers Squibb Laboratory, Billerica, Mass.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doria, A.S., Karshafian, R., Moineddin, R. et al. Contrast-enhanced triggered harmonic sonography for assessment of periarticular hemodynamic changes in experimental arthritis. Pediatr Radiol 36, 1242–1251 (2006). https://doi.org/10.1007/s00247-006-0300-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-006-0300-5