Abstract
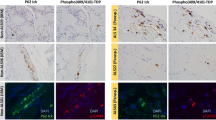
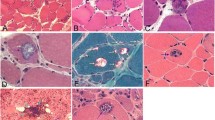
Accumulation of amyloid-β (Aβ) within muscle fibers has been considered an upstream step in the development of the s-IBM pathologic phenotype. Aβ42, which is considered more cytotoxic than Aβ40 and has a higher propensity to oligomerize, is preferentially increased in s-IBM muscle fibers. In Alzheimer disease (AD), low-molecular weight Aβ oligomers and toxic oligomers, also referred to as “Aβ-Derived Diffusible Ligands” (ADDLs), are considered strongly cytotoxic and proposed to play an important pathogenic role. ADDLs have been shown to be increased in AD brain. We now report for the first time that in s-IBM muscle biopsies Aβ-dimer, -trimer, and -tetramer are identifiable by immunoblots. While all the s-IBM samples we studied had Aβ-oligomers, their molecular weights and intensity varied between the patient samples. None of the control muscle biopsies had Aβ oligomers. Dot-immunoblots using highly specific anti-ADDL monoclonal antibodies also showed highly increased ADDLs in all s-IBM biopsies studied, while controls were negative. By immunofluorescence, in some of the abnormal s-IBM muscle fibers ADDLs were accumulated in the form of plaque-like inclusions, and were often increased diffusely in very small fibers. Normal and disease-controls were negative. By gold-immuno-electron microscopy, ADDL-immunoreactivities were in close proximity to 6–10 nm amyloid-like fibrils, and also were immunodecorating amorphous and floccular material. In cultured human muscle fibers, we found that inhibition of autophagy led to the accumulation of Aβ oligomers. This novel demonstration of Aβ42 oligomers in s-IBM muscle biopsy provides additional evidence that intra-muscle fiber accumulation of Aβ42 oligomers in s-IBM may contribute importantly to s-IBM pathogenic cascade.




Similar content being viewed by others
References
Askanas V, Engel WK (1992) Cultured normal and genetically abnormal human muscle. In: Rowland LP, Di Mauro S (eds) The handbook of clinical neurology, myopathies, vol 18. North Holland, Amsterdam, pp 85–116
Askanas V, Engel WK (2008) Inclusion-body myositis: muscle-fiber molecular pathology and possible pathogenic significance of its similarity to Alzheimer’s and Parkinson’s disease brains. Acta Neuropathol 116:583–595
Askanas V, Engel WK, Nogalska A (2009) Inclusion-body myositis: a degenerative muscle disease associated with intra-muscle-fiber multiprotein aggregates, proteasome inhibition, endoplasmic reticulum stress, and decreased lysosomal degradation. Brain Pathol 19:493–506
Askanas V, McFerrin J, Alvarez RB, Baque S, Engel WK (1997) Beta APP gene transfer into cultured human muscle induces inclusion-body myositis aspects. Neuroreport 8:2155–2158
Askanas V, McFerrin J, Baque S, Alvarez RB, Sarkozi E, Engel WK (1996) Transfer of beta-amyloid precursor protein gene using adenovirus vector causes mitochondrial abnormalities in cultured normal human muscle. Proc Natl Acad Sci USA 93:1314–1319
Barelli H, Lebeau A, Vizzavona J et al (1997) Characterization of new polyclonal antibodies specific for 40 and 42 amino acid-long amyloid beta peptides: their use to examine the cell biology of presenilins and the immunohistochemistry of sporadic Alzheimer’s disease and cerebral amyloid angiopathy cases. Mol Med 3:695–707
Chromy BA, Nowak RJ, Lambert MP et al (2003) Self-assembly of Abeta(1–42) into globular neurotoxins. Biochemistry 42:12749–12760
Dalakas MC (2010) Inflammatory muscle diseases: a critical review on pathogenesis and therapies. Curr Opin Pharmacol. doi:10.1016/j.coph.2010.03.001
De Strooper B (2010) Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiol Rev 90:465–494
El-Agnaf OM, Mahil DS, Patel BP, Austen BM (2000) Oligomerization and toxicity of beta-amyloid-42 implicated in Alzheimer’s disease. Biochem Biophys Res Commun 273:1003–1007
Engel WK, Askanas V (2006) Inclusion-body myositis: clinical, diagnostic and pathologic aspects. Neurology 66:S20–S29
Fratta P, Engel WK, McFerrin J, Davies KJ, Lin SW, Askanas V (2005) Proteasome inhibition and aggresome formation in sporadic inclusion-body myositis and in amyloid-{beta} precursor protein-overexpressing cultured human muscle fibers. Am J Pathol 167:517–526
Fukuchi K, Pham D, Hart M, Li L, Lindsey JR (1998) Amyloid beta deposition in skeletal muscle of transgenic mice: possible model of inclusion body myopathy. Am J Pathol 153:1687–1693
Gong Y, Chang L, Viola KL et al (2003) Alzheimer’s disease-affected brain: presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci USA 100:10417–10422
Gouras GK, Tampellini D, Takahashi RH, Capetillo-Zarate E (2010) Intraneuronal beta-amyloid accumulation and synapse pathology in Alzheimer’s disease. Acta Neuropathol 119:523–541
Ikezoe K, Furuya H, Arahata H et al (2009) Amyloid-beta accumulation caused by chloroquine injections precedes ER stress and autophagosome formation in rat skeletal muscle. Acta Neuropathol 117:575–582
Jin LW, Hearn MG, Ogburn CE et al (1998) Transgenic mice overexpressing the C-99 fragment of betaPP with an alpha-secretase site mutation develop a myopathy similar to human inclusion body myositis. Am J Pathol 153:1679–1686
Keller JN, Hanni KB, Markesbery WR (2000) Impaired proteasome function in Alzheimer’s disease. J Neurochem 75:436–439
Kitazawa M, Green KN, Caccamo A, LaFerla FM (2006) Genetically augmenting Abeta42 levels in skeletal muscle exacerbates inclusion body myositis-like pathology and motor deficits in transgenic mice. Am J Pathol 168:1986–1997
Kitazawa M, Vasilevko V, Cribbs DH, LaFerla FM (2008) Immunization with amyloid-beta attenuates inclusion body myositis-like myopathology and motor impairment in a transgenic mouse model. J Neurosci 29:6132–6141
Klein WL, Stine WB, Teplow DB (2004) Small assemblies of unmodified amyloid beta-protein are the proximate neurotoxin in Alzheimer’s disease. Neurobiol Aging 25:569–580
LaFerla FM, Green K, Oddo S (2007) Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci 8:499–509
Lambert MP, Barlow AK, Chromy BA et al (1998) Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA 95:6448–6453
Lambert MP, Velasco PT, Chang L et al (2007) Monoclonal antibodies that target pathological assemblies of Abeta. J Neurochem 100:23–35
Lindholm D, Wootz H, Korhonen L (2006) ER stress and neurodegenerative diseases. Cell Death Differ 13:385–392
Mirabella M, Alvarez RB, Bilak M, Engel WK, Askanas V (1996) Difference in expression of phosphorylated tau epitopes between sporadic inclusion-body myositis and hereditary inclusion-body myopathies. J Neuropathol Exp Neurol 55:774–786
Nixon RA (2007) Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci 120:4081–4091
Nogalska A, D’Agostino C, Terracciano C, Engel WK, Askanas V (2009) p62/SQSTM1 is overexpressed and prominently accumulated in inclusions of sporadic inclusion-body myositis muscle fibers, and can help differentiating it from polymyositis and dermatomyositis. Acta Neuropathol 118:407–413
Nogalska A, D’Agostino C, Terracciano C, Engel WK, Askanas V (2010) Impaired autophagy in sporadic inclusion-body myositis and in endoplasmic reticulum stress-provoked cultured human muscle fibers. Am J Pathol. doi:10.2353/ajpath.2010.100050
Nogalska A, Wojcik S, Engel WK, McFerrin J, Askanas V (2007) Endoplasmic reticulum stress induces myostatin precursor protein and NF-kappaB in cultured human muscle fibers: relevance to inclusion body myositis. Exp Neurol 204:610–618
Oddo S, Caccamo A, Tran L et al (2006) Temporal profile of amyloid-beta (Abeta) oligomerization in an in vivo model of Alzheimer disease. A link between Abeta and tau pathology. J Biol Chem 281:1599–1604
Shacka JJ, Klocke BJ, Shibata M et al (2006) Bafilomycin A1 inhibits chloroquine-induced death of cerebellar granule neurons. Mol Pharmacol 69:1125–1136
Terracciano C, Nogalska A, Engel WK, Askanas V (2010) In AbetaPP-overexpressing cultured human muscle fibers proteasome inhibition enhances phosphorylation of AbetaPP751 and GSK3beta activation: effects mitigated by lithium and apparently relevant to sporadic inclusion-body myositis. J Neurochem 112:389–396
Tomiyama T, Matsuyama S, Iso H et al (2010) A mouse model of amyloid beta oligomers: their contribution to synaptic alteration, abnormal tau phosphorylation, glial activation, and neuronal loss in vivo. J Neurosci 30:4845–4856
Tsuzuki K, Fukatsu R, Yoshida TakamaruY et al (1995) Amyloid beta protein in rat soleus muscle in chloroquine-induced myopathy using end-specific antibodies for A beta 40 and A beta 42: immunohistochemical evidence for amyloid beta protein. Neurosci Lett 202:77–80
Vattemi G, Engel WK, McFerrin J, Askanas V (2004) Endoplasmic reticulum stress and unfolded protein response in inclusion body myositis muscle. Am J Pathol 164:1–7
Vattemi G, Nogalska A, Engel WK, D’Agostino C, Checler F, Askanas V (2009) Amyloid-beta42 is preferentially accumulated in muscle fibers of patients with sporadic inclusion-body myositis. Acta Neuropathol 117:569–574
Acknowledgments
This study was supported by grants (to VA) from the National Institutes of Health (AG 16768 Merit Award), the Muscular Dystrophy Association, and the Helen Lewis Research Fund. Maggie Baburyan provided excellent technical assistance in electron microscopy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. Nogalska is on leave from the Department of Biochemistry, Medical University of Gdansk, Gdansk, Poland
Rights and permissions
About this article
Cite this article
Nogalska, A., D’Agostino, C., Engel, W.K. et al. Novel demonstration of amyloid-β oligomers in sporadic inclusion-body myositis muscle fibers. Acta Neuropathol 120, 661–666 (2010). https://doi.org/10.1007/s00401-010-0737-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-010-0737-3