Abstract
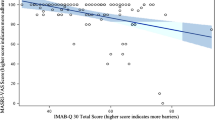
Although drug compliance is a crucial component of treatment effectiveness in chronic diseases, it has never been evaluated in patients with systemic scleroderma. Therefore, the aim of this descriptive study was to determine the drug compliance rate in systemic scleroderma patients and to identify risk factors for noncompliance in these patients. A cross-sectional observational study was conducted. All patients with systemic scleroderma (n = 41) who visited a rheumatic center and signed an informed consent form were included. Data were obtained during structured interviews with patients and from medical records. The Compliance Questionnaire Rheumatology (CQR) was used to determine patient compliance. The relationships between compliance rate and demographic and clinical characteristics were examined. The mean CQR score was 75 %. Based on a dichotomous rating, only 42 % of the patients achieved a satisfactory compliance rate (≥80 %). No relationships between various demographic and clinical characteristics and CQR score expressed as continuous or dichotomous variables were found. This study represents the first evaluation of drug compliance in patients with systemic scleroderma. Many noncompliant patients were identified, but no common risk factors for noncompliance were discovered. The reasons for noncompliance seem to depend on the personal features of the patients.
Similar content being viewed by others
References
Decuman S, Smith V, Verhaeghe S, Deschepper E, Vermeiren F, De Keyser F (2012) Work participation and work transition in patients with systemic sclerosis: a cross-sectional study. Rheumatology 51:297–304
Nguyen C, Poiraudeau S, Mestre-Stanislas C et al (2010) Employment status and socio-economic burden in systemic sclerosis: a cross-sectional survey. Rheumatology 49:1–8
Kowal-Bielecka O, Landewé R, Avouac J et al (2009) EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR). Ann Rheum Dis 68:620–628
Sabaté E (ed) (2003) Adherence to long-term therapies: Evidence for action. World Health Organization, Geneva
Pyne D, Chaabo K (2007) Adherence to immunosuppressant drugs in patients with connective tissue diseases [letter]. Rheumatology 46(12):1859
Garcia-Gonzalez A, Richardson M, Garcia Popa-Lisseanu M et al (2008) Treatment adherence in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Rheumatol 27(7):883–889
van den Bemt BJ, van den Hoogen FH, Benraad B, Hekster YA, van Riel PL, van Lankveld W (2009) Adherence rates and associations with nonadherence in patients with rheumatoid arthritis using disease modifying antirheumatic drugs. J Rheumatol 36:2164–2170
Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee (1980) Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 23:581–590
Fries JF, Spitz P, Kraines RG, Holman HR (1980) Measurement of patient outcome in arthritis. Arthritis Rheum 23:137–145
Ostojić P, Damjanov N (2006) The scleroderma assessment questionnaire (SAQ). Z Rheumatol 65:168–175
Silman A, Akesson A, Newman J et al (1998) Assessment of functional ability in patients with scleroderma: a proposed new disability assessment instrument. J Rheumatol 25:79–83
Ware JE Jr, Sherbourne CD (1992) The MOS 36 item Short-form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care 30:473–483
Ware J, Kosinski M, Dewey J (2000) How to score version two of the SF-36 Health Survey. QualityMetric, Lincoln
de Klerk E, van der Heijde D, van der Tempel H, van der Linden S (1999) Development of a questionnaire to investigate patient compliance with antirheumatic drug therapy. J Rheumatol 26:2635–2641
Beaton DE, Bombardier C, Guillemin F, Ferraz MB (2000) Guidelines for the process of cross-cultural adaptation of self-report measures. Spine 25:3186–3191
de Klerk E, van der Heijde D, Landewé R, van der Tempel H, van der Linden S (2003) The compliance-questionnaire-rheumatology compared with electronic medication event monitoring: a validation study. J Rheumatol 30:2469–2475
Streiner DL, Norman GR (1989) Health measurement scales. Oxford University Press, Oxford, p 175
Derk CT, Huaman G, Jimenez SA (2008) A retrospective randomly selected cohort study of D-penicillamine treatment in rapidly progressive diffuse cutaneous systemic sclerosis of recent onset. Brit J Dermatol 158:1063–1068
Hunzelmann N, Moinzadeh P, Genth E et al (2009) High frequency of corticosteroid and immunosuppressive therapy in patients with systemic sclerosis despite limited evidence for efficacy. Arthritis Res Ther 11(2):R30. doi:10.1186/ar2634
Pope JE, Ouimet JM, Krizova A (2006) Scleroderma treatment differs between experts and general rheumatologists. Arthrit Care Res 55(1):138–145
Meier FMP, Frommer KW, Dinser R et al (2012) Update on the profile of the EUSTAR cohort: an analysis of the EULAR Scleroderma Trials and Research group database. Ann Rheum Dis. doi:10.1136/annrheumdis-2011-200742
Denton CP, Krieg T, Guillevin L et al (2012) Demographic, clinical and antibody characteristics of patients with digital ulcers in systemic sclerosis: data from the DUO Registry. Ann Rheum Dis 71:718–721
Harrold LR, Andrade SE (2009) Medication adherence of patients with selected rheumatic conditions: a systematic review of the literature. Semin Arthritis Rheum 38(5):396–402
Vermeire E, Hearnshaw H, Van Royen P et al (2001) Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther 26(5):331–342
Neame R, Hammond A (2005) Beliefs about medications: a questionnaire survey of people with rheumatoid arthritis. Rheumatology 44:762–767
Sultan N, Pope JE, Clements PJ, Scleroderma Trials Study Group (2004) The Health Assessment Questionnaire (HAQ) is strongly predictive of good outcome in early diffuse scleroderma: results from an analysis of two randomized controlled trials in early diffuse scleroderma. Rheumatology 43(4):472–478
Pope JE, Bellamy N (1995) Sample size calculations in scleroderma: a rational approach to choosing outcome measurements in scleroderma trials. Clin Invest Med 18(1):1–10
Acknowledgments
This work was supported by a grant from Charles University in Prague (SVV 265 005).
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hromadkova, L., Soukup, T., Cermakova, E. et al. Drug compliance in patients with systemic scleroderma. Clin Rheumatol 31, 1577–1583 (2012). https://doi.org/10.1007/s10067-012-2050-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-012-2050-0