Abstract
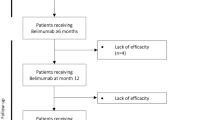
The objective of the study was to evaluate prospectively real-life experience on the effect of belimumab on patients with active systemic lupus erythematosus (SLE). Forty-eight patients with active SLE were evaluated after 1 year of continuous treatment. Thirty-eight patients were still on treatment at the end of 1 year, and it was possible to observe significant clinical improvement in the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score with a decrease from 12 ± 3.0 to 2.5 ± 2.5, also a decrease on the daily steroid dose from 30 ± 12.5 to 7.5 ± 5.0 mg and partial improvement on serology. Belimumab treatment is associated with real benefit in the majority of patients that maintain active disease in spite of continuing on standard of care.

Similar content being viewed by others
References
Kamal A, Khamashta M (2014) The efficacy of novel B cell biologics as the future of SLE treatment: a review. Autoimmun Rev 13:1094–1101
van Vollenhoven RF, Parodis I, Levitsky A (2013) Biologics in SLE: towards new approaches. Best Pract Res Clin Rheumatol 27:341–349
Vincent FB, Morand EF, Schneider P, Mackay F (2014) The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol 10:365–373
Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzová D, et al.; BLISS-76 Study Group. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011; 63:3918–30
Horowitz DL, Furie R (2012) Belimumab is approved by the FDA: what more do we need to know to optimize decision making? Curr Rheumatol Rep 14:318–323
Sciascia S, Talavera-Garcia E, Roccatello D, Baldovino S, Mengatti E, Cuadrado MJ (2015) Upcoming biological therapies in systemic lupus erythematosus. Int Immunopharmacol 27:189–193
Navarra SV, Guzman RM, Gallagher AE, Hall S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, Leon MG, Tanosescu T, Nasonov E, Lan JL, Pineda L, Zhong ZJ, Freimuth W, Petri MA (2011) Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised placebo-controlled, phase 3 trial. Lancet 377:721–781
Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O, Sturfelt G, Ramsey-Goldman R, Bae SC, Hanly JG, Sánchez-Guerrero J, Clarke A, Aranow C, Manzi S, Urowitz M, Gladman D, Kalunian K, Costner M, Werth VP, Zoma A, Bernatsky S, Ruiz-Irastorza G, Khamashta MA, Jacobsen S, Buyon JP, Maddison P, Dooley MA, van Vollenhoven RF, Ginzler E, Stoll T, Peschken C, Jorizzo JL, Callen JP, Lim SS, Fessler BJ, Inanc M, Kamen DL, Rahman A, Steinsson K, Franks AG Jr, Sigler L, Hameed S, Fang H, Pham N, Brey R, Weisman MH, McGwin G Jr, Magder LS (2012) Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64(8):2677–2686
Touma Z, Urowitz MB, Gladman DD (2010) SLEDAI-2K for a 30-day window. Lupus 19:49–51
Ginzler EM, Wallace DJ, Merrill JT, Furie RA, Stohl W, Chatham WW et al (2014) Disease control and safety of belimumab plus standard therapy over 7 years in patients with systemic lupus erythematosus. J Rheumatol 41:300–309
Hui-Yuen J, Taylor J, Li XQ, Bermudez L, Isgro J, Eichenfield A (2014) Favorable response to belimumab in pediatric onset systemic lupus erythematosus. Arthritis Rheumatol 66:S293
Collins C, Dall M, Era C, Macahiling C, Molta C, Kan H, Kolscieny V et al (2015) Belimumab 24-month treatment outcomes in patients with systemic lupus erythematosus (SLE) high disease activity: results from the OBSERrve real-world study. Clin Exp Rheumatol 33:3(S).
Parodis I, Sjöwall C, Jönsen A, Zickert A, Frodlund M, Ramsköld D et al (2015) Decreased disease activity and corticosteroid usage and improved quality of life during belimumab treatment in patients with systemic lupus erythematosus—a prospective real-life observational study [abstract]. Arthritis Rheumatol :suppl 10:67
Askanase AD, Yazdany J, Molta CT (2014) Post-marketing experiences with belimumab in the treatment of SLE patients. Rheum Dis Clin North Am 40:507–517
Hui-Yuen JS, Li XQ, Askanase AD (2015) Belimumab in systemic lupus erythematosus: a perspective review. Ther Adv Musculoskelet Dis 7:115–121
Andreoli L, Reggia R, Pea L, Frassi M, Zanola A, Cartella S et al (2014) Belimumab for the treatment of refractory systemic lupus erythematosus: real-life experience in the first year of use in 18 Italian patients. Isr Med Assoc J 10:651–653
Hui-Yuen JS, Reddy A, Taylor J, Li X, Eichenfield AH, Bermudez LM et al (2015) Safety and efficacy of belimumab to treat systemic lupus erythematosus in academic clinical practices. J Rheumatol 42:2288–2295
van Vollenhoven RF, Petri MA, Cervera R, Roth DA, Ji BN, Kleondis CS et al (2012) Belimumab in the treatment of systemic lúpus erythematosus: high disease actvity predictors of response. Annal Rheumatic Dis 7:1343–1349
Muangchan C, van Vollenhoven RF, Bernatsky SR, Smith CD, Hudson M, Inanç M et al (2015) Treatment algorithms in systemic lupus erythematosus. Arthritis Care Res (Hoboken). 67(9):1237–1245
Scheinberg M, Goldenberg J, Feldman D, Nobrega JL (2008) Retrospective study evaluating dose standards for infliximab in patients with rheumatoid arthritis at Hospital Israelita Albert Einstein. Clin Rheumatol 27:1049–1053
Golmia RP, Scheinberg M (2013) Retention rates of infliximab and tocilizumab during a three year period. Einstein 11:492–494
Yuen HK, Cunningham MA (2014) Optimal management of fatigue in patients with systemic lupus erythematosus: a systematic review. Optimal management of fatiguein patients with systemic lupus erythematosus: a systematic review. Ther Clin Risk Manag 10:775–786
Scheinberg M, Golmia R (2014) Real life experience on the effect of belimumab in patients with active systemic lupus. Springerplus 22:758–760
Furie R, Petri MA, Strand V, Gladman DD, Zhong ZJ, Freimuth WW, BLISS-52 and BLISS-76 Study Groups (2014) Clinical, laboratory and health-related quality of life correlates of systemic lupus erythematosus responder index response: a post hoc analysis of the phase 3 belimumab trials. Lupus Sci Med J 26;1
Dooley MA, Houssiau F, Aranow C, D’Cruz DP, Askanase A, Roth DA, Zhong ZJ, Cooper S, Freimuth WW, Ginzler EM, BLISS-52 and −76 Study Groups (2013) Effect of belimumab treatment on renal outcomes: results from the phase 3 belimumab clinical trials in patients with SLE. Lupus 22:63–72
Hiepe WF, Latinis KM, Thomas M, Scheinberg MA, Clarke A, BLISS-52 Study Group; BLISS-76 Study Group et al (2012) Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis Rheum 64:2328–2335
Van Vollenhoven RF, Petri M, Wallace DJ, Roth D, Molta CT, Hammer AE, Tang Y, Thompson A (2016) Arthritis Rheumatol. doi:10.1002/art.39682
Acknowledgments
This research received funds from the Federico Foundation to one of the authors (M.A.S).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the respective IRB of each center.
Disclosures
None.
Additional information
M.A. Scheinberg, MDPhD, Internist and Rheumatologist, Hospital Israelita Albert Einstein and Hospital AACD Clinical Research Division, State of São Paulo, Brazil; F.F.N. de Melo, MD, Rheumatologist, Hospital Unimed Volta Redonda, State of Rio de Janeiro, Brazil; A.N. Bueno, MD, Rheumatologist, Professor of Rheumatology University of Alfenas, Centro Médico de Varginha, Hospital Humanitas, State of Minas Gerais, Brazil; C. M. Costa, MD, Rheumatologist, Centro Médico de Varginha, Hospital Humanitas, State of Minas Gerais, Brazil; M.L.A.A. Bahr, MD, Rheumatologist, Centro Médico de Varginha, Hospital Humanitas, State of Minas Gerais, Brazil; E.R. Reis, MD, Rheumatologist, Centro Médico de Varginha, Hospital Humanitas, State of Minas Gerais, Brazil
Rights and permissions
About this article
Cite this article
Scheinberg, M., de Melo, F.F.N., Bueno, A.N. et al. Belimumab for the treatment of corticosteroid-dependent systemic lupus erythematosus: from clinical trials to real-life experience after 1 year of use in 48 Brazilian patients. Clin Rheumatol 35, 1719–1723 (2016). https://doi.org/10.1007/s10067-016-3268-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-016-3268-z