Abstract
Several solutes (e.g., growth factors, cationic solutes, etc.) can reversibly bind to the extracellular matrix (ECM) of biological tissues. Binding interactions have significant implications on transport of such solutes through the ECM. In order to fully delineate transport phenomena in biological tissues, knowledge of binding kinetics is crucial. In this study, a new method for the simultaneous determination of solute anisotropic diffusivity and binding reaction rates was presented. The new technique was solely based on Fourier analysis of fluorescence recovery after photobleaching (FRAP) images. Computer-simulated FRAP tests were used to assess the sensitivity and the robustness of the method to experimental parameters, such as anisotropic solute diffusivity and rates of binding reaction. The new method was applied to the determination of diffusivity and binding rates of 5-dodecanoylaminofluorescein (DAF) in bovine coccygeal annulus fibrosus (AF). Our findings indicate that DAF reversibly binds to the ECM of AF. In addition, it was found that DAF diffusion in AF is anisotropic. The results were in agreement with those reported in previous studies. This study provides a new tool for the simultaneous determination of solute anisotropic diffusion tensor and rates of binding reaction that can be used to investigate diffusive–reactive transport in biological tissues and tissue engineered constructs.






Similar content being viewed by others
References
Arkill, K. P., and C. P. Winlove. Solute transport in deep and calcified zones of articular cartilage. Osteoarthr. Cartil. 13:708–714, 2008.
Berk, D. A., F. Yuan, M. Leunig, and R. K. Jain. Fluorescence photobleaching with spatial Fourier analysis: measurement of diffusion in light-scattering media. Biophys. J. 65:2428–2436, 1993.
Bhakta, N. R., A. M. Garcia, E. H. Frank, A. J. Grodzinsky, and T. I. Morales. The insulin-like growth factors (IGFs) I and II bind to articular cartilage via the IGF-binding proteins. J. Biol. Chem. 275:5860–5866, 2000.
Braga, J., J. G. McNally, and M. Carmo-Fonseca. A reaction-diffusion model to study RNA motion by quantitative fluorescence recovery after photobleaching. Biophys. J. 92:2694–2703, 2007.
Bulinski, J. C., D. J. Odde, B. J. Howell, T. D. Salmon, and C. M. Waterman-Storer. Rapid dynamics of the microtube binding of ensconsin in vivo. J. Cell Sci. 114:3885–3897, 2001.
Burstein, D., M. L. Gray, A. L. Hartman, R. Gipe, and B. D. Foy. Diffusion of small solutes in cartilage as measured by nuclear magnetic resonance (NMR) spectroscopy and imaging. J. Orthop. Res. 11:465–478, 1993.
Carrero, G., D. McDonald, E. Crawford, G. de Vries, and M. J. Hendzel. Using FRAP and mathematical modeling to determine the in vivo kinetics of nuclear proteins. Methods 29:14–28, 2003.
Carrero, G., E. Crawford, M. J. Hendzel, and G. de Vries. Characterizing fluorescence recovery curves for nuclear proteins undergoing binding events. Bull. Math. Biol. 66:1515–1545, 2004.
Chiu, E. J., D. C. Newitt, M. R. Segal, S. S. Hu, J. C. Lotz, and S. Majumdar. Magnetic resonance imaging measurement of relaxation and water diffusion in the human lumbar intervertebral disc under compression in vitro. Spine 26:E437–E444, 2001.
Coscoy, S., F. Waharte, A. Gautreau, M. Martin, D. Louverd, P. Mangeat, M. Arpin, and F. Amblard. Molecular analysis of microscopic ezrin dynamics by two-photon FRAP. Proc. Natl Acad. Sci. 99:12813–12818, 2002.
Crank, J. Diffusion and chemical reaction. In: The Mathematics of Diffusion. New York: Oxford University Press Inc., 1975, pp. 338–340.
Diamant, B., J. Karlsson, and A. Nachemeson. Correlation between lactate levels and pH in discs of patients with lumbar rhizopathies. Experientia 24:1195–1196, 1968.
Dundr, M., U. Hoffmann-Rohrer, Q. Hu, I. Grummt, L. I. Rothblum, R. D. Phair, and T. Misteli. A kinetic framework for a mammalian RNA polymerase in vivo. Science 298:1623–1626, 2002.
Fung, Y. C. Quasi-linear viscoelasticity of soft tissues. In: Biomechanics: Mechanical Properties of Living Tissues. New York: Springer-Verlag New York, Inc., 1993, pp. 279–280.
Garcia, A. M., N. Szasz, S. B. Trippel, T. I. Morales, A. J. Grodzinsky, and E. H. Frank. Transport and binding of insulin-like growth factor I through articular cartilage. Arch. Biochem. Biophys. 415:69–79, 2003.
Hsu, E. W., and L. A. Setton. Diffusion tensor microscopy of the intervertebral disc anulus fibrosus. Magn. Reson. Med. 41:992–999, 1999.
Iatridis, J. C., and I. ap Gwynn. Mechanisms for mechanical damage in the intervertebral disc annulus fibrosus. J. Biomech. 37:1165–1175, 2004.
Inagawa, K., T. Oohashi, K. Nishida, J. Minaguchi, T. Tsubakishita, K. O. Yaykasli, A. Ohtsuka, T. Ozaki, T. Moriguchi, and Y. Ninomiya. Optical imaging of mouse articular cartilage using the glycosaminoglycans binding property of fluorescent-labeled octaarginine. Osteoarthr. Cartil. 17:1209–1218, 2009.
Jackson, A. R., and W. Y. Gu. Transport properties of cartilaginous tissues. Curr. Rheumatol. Rev. 5:40–50, 2009.
Jackson, A. R., H. Yao, M. D. Brown, and W. Y. Gu. Anisotropic ion diffusivity in intervertebral disc: an electrical conductivity approach. Spine 31:2783–2789, 2006.
Jackson, A. R., T. Y. Yuan, C. Y. Huang, F. Travascio, and W. Y. Gu. Effect of compression and anisotropy on the diffusion of glucose in annulus fibrosus. Spine 33:1–7, 2008.
Kang, M., and A. K. Kenworthy. A closed-form analytic expression for FRAP formula for the binding diffusion model. Biophys. J. 95:L13–L15, 2008.
Kaufman, E. N., and R. K. Jain. Quantification of transport and binding parameters using fluorescence recovery after photobleaching. Biophys. J. 58:873–885, 1990.
Kaufman, E. N., and R. K. Jain. Measurement of mass transport and reaction parameters in bulk solution using photobleaching. Biophys. J. 60:596–610, 1991.
Kimura, H., K. Sugaya, and P. R. Cook. The transcription cycle of RNA polymerase II in living cells. J. Cell Biol. 159:457–459, 2002.
Leddy, H. A., and F. Guilak. Site-specific molecular diffusion in articular cartilage measured using fluorescence recovery after photobleaching. Ann. Biomed. Eng. 31:753–760, 2003.
Leddy, H. A., and F. Guilak. Site-specific effects of compression on macromolecular diffusion in articular cartilage. Biophys. J. 95:4890–4895, 2008.
Leddy, H. A., H. A. Awad, and F. Guilak. Molecular diffusion in tissue-engineered cartilage constructs: effects of scaffold material, time, and culture conditions. J. Biomed. Mater. Res. B Appl. Biomater. 70:397–406, 2004.
Leddy, H. A., M. A. Haider, and F. Guilak. Diffusional anisotropy in collagenous tissues: fluorescence imaging of continuous point photobleaching. Biophys. J. 91:311–316, 2006.
Lele, T. P., and D. E. Ingber. A mathematical model to determine molecular kinetic rate constants under non-steady state conditions using fluorescence recovery after photobleaching (FRAP). Biophys. Chem. 120:32–35, 2006.
Lippincott-Schwartz, J., N. Altan-Bonnet, and G. H. Patterson. Photobleaching and photoactivation: following protein dynamics in living cells. Nat. Cell Biol. Supplement:S7–S13, 2003.
Maroudas, A. Distribution and diffusion of solutes in articular cartilage. Biophys. J. 10:365–379, 1970.
Maroudas, A. Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology 12:233–248, 1975.
Meyvis, T. K. L., S. C. De Smedt, P. Van Oostveldt, and J. Demeester. Fluorescence recovery after photobleaching: a versatile tool for mobility and interaction measurements in pharmaceutical research. Pharm. Res. 16:1153–1162, 1999.
Ohshima, H., H. Tsuji, N. Hiarano, H. Ishihara, Y. Katoh, and H. Yamada. Water diffusion pathway, swelling pressure, and biomechanical properties of the intervertebral disc during compression load. Spine 14:1234–1244, 1989.
Perry, R. H. Mathematical tables and mathematics. In: Engineering Manual, edited by R. H. Perry, D. W. Green, and J. O. Maloney. New York: McGraw Hill, Inc., 1976, pp. 78–80.
Quinn, T. M., P. Kocian, and J. J. Meister. Static compression is associated with decreased diffusivity of dextrans in cartilage explants. Arch. Biochem. Biophys. 384:327–334, 2000.
Quinn, T. M., V. Morel, and J. J. Meister. Static compression of articular cartilage can reduce solute diffusivity and partitioning: implications for the chondrocyte biological response. J. Biomech. 34:1463–1469, 2001.
Reits, E. A. J., and J. J. Neefies. From fixed to FRAP: measuring protein mobility and activity in living cells. Nat. Cell Biol. 3:E145–E147, 2001.
Schneiderman, R., E. Snir, O. Popper, J. Hiss, H. Stein, and A. Maroudas. Insulin-like growth factor-I and its complexes in normal human articular cartilage: studies of partition and diffusion. Arch. Biochem. Biophys. 324(1):159–172, 1995.
Sprague, B. L., and J. G. McNally. FRAP analysis of binding: proper and fitting. Trends Cell Biol. 15:84–91, 2005.
Sprague, B. L., R. L. Pego, D. A. Stavreva, and J. G. McNally. Analysis of binding reactions by fluorescence recovery after photobleaching. Biophys. J. 86:3473–3495, 2004.
Torzilli, P. A., T. C. Adams, and R. J. Mis. Transient solute diffusion in articular cartilage. J. Biomech. 20:203–214, 1987.
Torzilli, P. A., D. A. Grande, and J. M. Arduino. Diffusive properties of immature articular cartilage. J. Biomed. Mater. Res. 40:132–138, 1998.
Travascio, F., and W. Y. Gu. Anisotropic diffusive transport in annulus fibrosus: experimental determination of the diffusion tensor by FRAP technique. Ann. Biomed. Eng. 35:1739–1748, 2007.
Travascio, F., A. R. Jackson, M. D. Brown, and W. Y. Gu. Relationship between solute transport properties and tissue morphology in human annulus fibrosus. J. Orthop. Res. 27:1625–1630, 2009.
Travascio, F., W. Zhao, and W. Y. Gu. Characterization of anisotropic diffusion tensor of solute in tissue by video-FRAP imaging technique. Ann. Biomed. Eng. 37:813–823, 2009.
Tsay, T. T., and K. Jacobson. Spatial Fourier analysis of video photobleaching measurements. Principles and optimization. Biophys. J. 60:360–368, 1991.
Tsibidis, G. D. Quantitative interpretation of binding reactions of rapidly diffusing species using fluorescence recovery after photobleaching. J. Microscopy 233:384–390, 2009.
Urban, J. P. G., S. Holms, A. Maroudas, and A. Nachemson. Nutrition of the intervertebral disc: an in vivo study of solute transport. Clin. Orthop. 129:101–114, 1977.
Urban, J. P., S. Holm, and A. Maroudas. Diffusion of small solutes into the intervertebral disc: as in vivo study. Biorheology 15:203–221, 1978.
Urban, J. P., S. Holm, A. Maroudas, and A. Nachemson. Nutrition of the intervertebral disc: effect of fluid flow on solute transport. Clin. Orthop. 170:296–302, 1982.
Urban, J. P., S. Smith, and J. C. Fairbank. Nutrition of the intervertebral disc. Spine 29:2700–2709, 2004.
Zhang, L., B. S. Gardiner, D. W. Smith, P. Pivonka, and A. J. Grodzinsky. The effect of cyclic deformation and solute binding on solute transport in cartilage. Arch. Biochem. Biophys. 457:47–56, 2007.
Acknowledgments
This project was supported by the grants from the National Institute of Arthritis, Musculoskeletal and Skin Diseases (AR050609), and the National Institute of Biomedical Imaging and Bioengineering (EB008653).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Sean S. Kohles oversaw the review of this article.
Appendix
Appendix
Dimensionless Analysis of the Field Equations for Diffusive–reactive Transport
Now, for the sake of simplicity, the ideal case of isotropic diffusive–reactive fluorescence recovery is considered. The extension of this analysis to the more general case of anisotropic diffusion can be easily derived.
In the case of isotropic diffusive–reactive transport, recovery of fluorescence intensity within the images is controlled by four parameters: the diffusion coefficient D, the pseudo-rate of binding association \( k_{a}^{*} \), the rate of binding dissociation k d , and the initial diameter of the bleach spot (d). Let Da and R be two dimensionless numbers defined as42
The number Da represents the ratio of the diffusion time to the characteristic time of binding association, and, therefore, has the physical meaning of the Damkhöler number.36 The number R represents the ratio of the characteristic time of binding dissociation to that of binding association. Let d be the characteristic length and τ the characteristic time of the diffusive–reactive process, defined as
Equations (3a–b) can be rewritten in the following dimensionless form:
where \( \bar{c}^{f} \) and \( \bar{c}^{b} \) are the concentrations of free and bound solutes normalized by the total pre-bleach concentration of fluorescent solute; \( \hat{t} \)(defined as \( \hat{t} \) = t/τ) and \( \hat{\nabla }^{2} \) (defined as \( \hat{\nabla }^{2} \) = \( \nabla^{2} \)·d 2) are the dimensionless time and the dimensionless Laplacian operator, respectively. Eqs. (A2a–b) indicate that the two dimensionless numbers, Da and R, govern the diffusive–reactive fluorescence recovery.
Solution of the Diffusion–Reaction Equations in the Fourier Space
The system of Eqs. (3a–b) is transformed in the 2D Fourier space defined by the dimensionless frequencies (u, v):
where C f and C b are the 2D Fourier transforms of the concentrations of free and bound solutes, normalized with respect to the pre-bleach total concentration of solute (free + bound); L is the dimension of the video-FRAP image, corresponding to eight times the value of the initial diameter of the bleach spot (d) used in the experiments. The function D(ξ), defined as47
with
includes the components of the 2D diffusion tensor D (i.e., D xx , D xy , and D yy ). Assuming that, before bleaching (t = 0), the system is at equilibrium (both dC f /dt and dC b /dt equal zero), from Eqs. (A3b) we have
Since C f (u, v, 0) + C b (u, v, 0) = 1, it follows that
Equations (A7a–b) represent the initial conditions for Eqs. (3a–b). Note that, in the Fourier space, the intensity of the fluorescence emission is proportional to the total concentration of the fluorescent solute (C = C f + C b ) according to the following relationship2:
Combining the solution of Eqs. (3a–b) with (A8), it follows that
where
Curve-fitting the 2D Fourier transform of the fluorescence emission of a video-FRAP image series with (A9) yields the binding rates \( k_{a}^{*} \) and k d , together with D(ξ).
Determination of the Principal Components of the 2D Anisotropic Diffusion Tensor
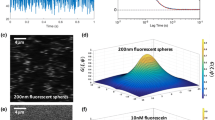
Let (x, y) stand for the fixed coordinate system of the microscope, and (x′, y′) be the principal directions of D (i.e., material coordinate system), see Fig. 7a. The components of D in the fixed coordinate system (i.e., D xx , D xy , and D yy ) are calculated by curve-fitting the experimental values of D(ξ), estimated at points (u, v) describing an arc of circumference (u 2 + v 2 = constant) in the Fourier space spanning from 0 to π, with Eq. (A4) (Figs. 7b–7c). The components D xx , D xy , and D yy are related to the principal components of D (\( D^{\prime}_{xx} \) and \( D^{\prime}_{yy} \)) by the following relationship:
(a) The orientation of the material coordinate system (x′, y′) with respect to the fixed coordinate system (x, y) is θ. (b) D(ξ) is defined over an arc of circumference (u 2 + v 2 = constant) in the Fourier space spanning from 0 to π. (c) Representative curve-fitting of the values of D(ξ) (open circles) with Eq. (A4) (solid line) to yield D xx , D xy , and D yy . Note that D(ξ) was determined by curve-fitting of computer-simulated FRAP data (D xx = 5 × 10−7 cm2 s−1, D xy = 1.667 × 10−7 cm2 s−1, D yy = 5 × 10−7 cm2 s−1, Da = 500, and R = 1) with Eq. (6)
where θ is the orientation of the principal directions of D with respect to (x,y) (Fig. 7a). It follows that
Rights and permissions
About this article
Cite this article
Travascio, F., Gu, W.Y. Simultaneous Measurement of Anisotropic Solute Diffusivity and Binding Reaction Rates in Biological Tissues by FRAP. Ann Biomed Eng 39, 53–65 (2011). https://doi.org/10.1007/s10439-010-0138-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-010-0138-8