Abstract
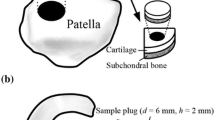
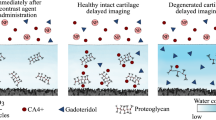
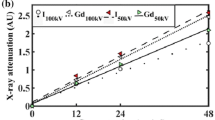
Solute transport through the extracellular matrix (ECM) is crucial to chondrocyte metabolism. Cartilage injury affects solute transport in cartilage due to alterations in ECM structure and solute-matrix interactions. Therefore, cartilage injury may be detected by using contrast agent-based clinical imaging. In the present study, effects of mechanical injury on transport of negatively charged contrast agents in cartilage were characterized. Using cartilage plugs injured by mechanical compression protocol, effective partition coefficients and diffusion fluxes of iodine- and gadolinium-based contrast agents were measured using high resolution microCT imaging. For all contrast agents studied, effective diffusion fluxes increased significantly, particularly at early times during the diffusion process (38 and 33% increase after 4 min, P < 0.05 for iodine and Gd-DTPA; and 76% increase after 10 min for diatrizoate, P < 0.05). Effective partition coefficients were unaffected in mechanically injured cartilage. Mechanical injury reduced PG content and collagen integrity in cartilage superficial zone. This study suggests that alterations in contrast agent diffusion flux, a non-equilibrium transport parameter, provides a more sensitive indicator for assessment of cartilage matrix integrity than partition coefficient and the equilibrium distribution of solute. These findings may help in developing clinical methods of contrast agent-based imaging to detect cartilage injury.






Similar content being viewed by others
References
Arokoski, J. P., M. M. Hyttinen, T. Lapvetelainen, P. Takacs, B. Kosztaczky, L. Modis, et al. Decreased birefringence of the superficial zone collagen network in the canine knee (stifle) articular cartilage after long distance running training, detected by quantitative polarised light microscopy. Ann. Rheum. Dis. 55:253–264, 1996.
Bansal, P. N., N. S. Joshi, V. Entezari, M. W. Grinstaff, and B. D. Snyder. Contrast enhanced computed tomography can predict the glycosaminoglycan content and biomechanical properties of articular cartilage. Osteoarthr. Cartil. 18:184–191, 2010.
Bashir, A., M. L. Gray, J. Hartke, and D. Burstein. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn. Reson. Med. 41:857–865, 1999.
Chin, H. C., M. Moeini, and T. M. Quinn. Solute transport across the articular surface of injured cartilage. Arch. Biochem. Biophys. 535:241–247, 2013.
Choi, J. A., and G. E. Gold. MR imaging of articular cartilage physiology. Magn. Reson. Imaging Clin. N. Am. 19:249–282, 2011.
Crema, M. D., F. W. Roemer, M. D. Marra, D. Burstein, G. E. Gold, F. Eckstein, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics 31:37–61, 2011.
Evans, R. C., and T. M. Quinn. Solute diffusivity correlates with mechanical properties and matrix density of compressed articular cartilage. Arch. Biochem. Biophys. 442:1–10, 2005.
Flik, K. R., N. Verma, B. J. Cole, and R. R. Bach. Articular cartilage: structure, biology, and function. In: Cartilage Repair Strategies, edited by R. J. I. Williams. Totowa, NJ: Humana Press, 2007, pp. 1–12.
Garcia, A. M., E. H. Frank, P. E. Grimshaw, and A. J. Grodzinsky. Contributions of fluid convection and electrical migration to transport in cartilage: relevance to loading. Arch. Biochem. Biophys. 333:317–325, 1996.
Hawezi, Z. K., E. Lammentausta, J. Svensson, L. E. Dahlberg, and C. J. Tiderius. In vivo transport of Gd-DTPA(2-) in human knee cartilage assessed by depth-wise dGEMRIC analysis. J. Magn. Reson. Imaging 34:1352–1358, 2011.
Jackson, A., and W. Gu. Transport properties of cartilaginous tissues. Curr. Rheumatol. Rev. 5:40, 2009.
Joshi, N. S., P. N. Bansal, R. C. Stewart, B. D. Snyder, and M. W. Grinstaff. Effect of contrast agent charge on visualization of articular cartilage using computed tomography: exploiting electrostatic interactions for improved sensitivity. J. Am. Chem. Soc. 131:13234–13235, 2009.
Kallioniemi, A. S., J. S. Jurvelin, M. T. Nieminen, M. J. Lammi, and J. Töyräs. Contrast agent enhanced pQCT of articular cartilage. Phys. Med. Biol. 52:1209–1219, 2007.
Kiraly, K., T. Lapvetelainen, J. Arokoski, K. Torronen, L. Modis, I. Kiviranta, et al. Application of selected cationic dyes for the semiquantitative estimation of glycosaminoglycans in histological sections of articular cartilage by microspectrophotometry. Histochem. J. 28:577–590, 1996.
Kokkonen, H. T., A. S. Aula, H. Kröger, J. S. Suomalainen, E. Lammentausta, E. Mervaala, J. S. Jurvelin, and J. Töyräs. Delayed computed tomography arthrography of human knee cartilage in vivo. Cartilage 3(4):334–341, 2012.
Kokkonen, H. T., J. S. Jurvelin, V. Tiitu, and J. Töyräs. Detection of mechanical injury of articular cartilage using contrast enhanced computed tomography. Osteoarthr. Cartil. 19:295–301, 2011.
Kokkonen, H. T., J. S. Suomalainen, A. Joukainen, H. Kröger, J. Sirola, J. S. Jurvelin, J. Salo, and J. Töyräs. In vivo diagnostics of human knee cartilage lesions using delayed CBCT arthrography. J. Orthop. Res. 32(3):403–412, 2014.
Krause, W., and P. W. Schneider. Chemisry of X-Ray Contrast Agents: Contrast Agents II. Berlin: Springer, pp. 107–150, 2002.
Kulmala, K. A., R. K. Korhonen, P. Julkunen, J. S. Jurvelin, T. M. Quinn, H. Kroger, et al. Diffusion coefficients of articular cartilage for different CT and MRI contrast agents. Med. Eng. Phys. 32:878–882, 2010.
Kurz, B., M. Jin, P. Patwari, D. M. Cheng, M. W. Lark, and A. J. Grodzinsky. Biosynthetic response and mechanical properties of articular cartilage after injurious compression. J. Orthop. Res. 19:1140–1146, 2001.
Lu, X. L., and V. C. Mow. Biomechanics of articular cartilage and determination of material properties. Med. Sci. Sports Exerc. 40:193–199, 2008.
Maroudas, A. Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology 12:233–248, 1975.
Maroudas, A. Transport of solutes through cartilage: permeability to large molecules. J. Anat. 122:335–347, 1976.
McKenzie, C. A., A. Williams, P. V. Prasad, and D. Burstein. Three-dimensional delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) at 1.5T and 3.0T. J. Magn. Reson. Imaging 24:928–933, 2006.
Morel, V., C. Berutto, and T. M. Quinn. Effects of damage in the articular surface on the cartilage response to injurious compression in vitro. J. Biomech. 39:924–930, 2006.
Morel, V., and T. M. Quinn. Cartilage injury by ramp compression near the gel diffusion rate. J. Orthop. Res. 22:145–151, 2004.
Morel, V., and T. M. Quinn. Short-term changes in cell and matrix damage following mechanical injury of articular cartilage samples and modelling of microphysical mediators. Biorheology 41:509–519, 2004.
Palmer, A. W., R. E. Guldberg, and M. E. Levenston. Analysis of cartilage matrix fixed charge density and three-dimensional morphology via contrast-enhanced microcomputed tomography. Proc. Natl. Acad. Sci. USA 103:19255–19269, 2006.
Pan, J., X. Zhou, W. Li, J. E. Novotny, S. B. Doty, and L. Wang. In situ measurement of transport between subchondral bone and articular cartilage. J. Orthop. Res. 27(10):1347–1352, 2009.
Pearle, A. D., R. F. Warren, and S. A. Rodeo. Basic science of articular cartilage and osteoarthritis. Clin. Sports Med. 24:1–12, 2005.
Quinn, T. M., R. G. Allen, B. J. Schalet, P. Perumbuli, and E. B. Hunziker. Matrix and cell injury due to sub-impact loading of adult bovine articular cartilage samples: effects of strain rate and peak stress. J. Orthop. Res. 19:242–249, 2001.
Rieppo, J., J. Hallikainen, J. S. Jurvelin, I. Kiviranta, H. J. Helminen, and M. M. Hyttinen. Practical considerations in the use of polarized light microscopy in the analysis of the collagen network in articular cartilage. Microsc. Res. Tech. 71:279–287, 2008.
Salo, E. N., M. J. Nissi, K. A. Kulmala, V. Tiitu, J. Töyräs, and M. T. Nieminen. Diffusion of Gd-DTPA(2)(-) into articular cartilage. Osteoarthr. Cartil. 20:117–126, 2012.
Samosky, J. T., D. Burstein, W. Eric Grimson, R. Howe, S. Martin, and M. L. Gray. Spatially-localized correlation of dGEMRIC-measured GAG distribution and mechanical stiffness in the human tibial plateau. J. Orthop. Res. 23:93–101, 2005.
Silvast, T. S., H. T. Kokkonen, J. S. Jurvelin, T. M. Quinn, M. T. Nieminen, and J. Töyräs. Diffusion and near-equilibrium distribution of MRI and CT contrast agents in articular cartilage. Phys. Med. Biol. 54:6823–6836, 2009.
Stockwell, R. A. Biology of the Cartilage Cells. Cambridge: Cambridge University Press, 1979.
Tiderius, C. J., L. E. Olsson, P. Leander, O. Ekberg, and L. Dahlberg. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) in early knee osteoarthritis. Magn. Reson. Med. 49:488–492, 2003.
Torzilli, P. A., J. M. Arduino, J. D. Gregory, and M. Bansal. Effect of proteoglycan removal on solute mobility in articular cartilage. J. Biomech. 30:895–902, 1997.
Verteramo, A., and B. B. Seedhom. Effect of a single impact loading on the structure and mechanical properties of articular cartilage. J. Biomech. 40:3580–3589, 2007.
Wayne, J. S., K. A. Kraft, K. J. Shields, C. Yin, J. R. Owen, and D. G. Disler. MR imaging of normal and matrix-depleted cartilage: correlation with biomechanical function and biochemical composition. Radiology 228:493–499, 2003.
Xie, L., A. S. Lin, R. E. Guldberg, and M. E. Levenston. Nondestructive assessment of sGAG content and distribution in normal and degraded rat articular cartilage via EPIC-microCT. Osteoarthr. Cartil. 18:65–72, 2010.
Yoo, H. J., S. H. Hong, J. Y. Choi, I. J. Lee, S. J. Kim, J. A. Choi, et al. Contrast-enhanced CT of articular cartilage: experimental study for quantification of glycosaminoglycan content in articular cartilage. Radiology 261:805–812, 2011.
Acknowledgments
Academy of Finland is acknowledged for funding (Decision Number 269315).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Karol Miller oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Kokkonen, H.T., Chin, H.C., Töyräs, J. et al. Solute Transport of Negatively Charged Contrast Agents Across Articular Surface of Injured Cartilage. Ann Biomed Eng 45, 973–981 (2017). https://doi.org/10.1007/s10439-016-1756-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-016-1756-6