Abstract
Background
Limited data are available on long-term clinical outcomes regarding the switch from Remicade® to the infliximab biosimilar CT-P13 in inflammatory bowel disease (IBD) patients.
Aims
To investigate long-term efficacy, safety, pharmacokinetic profile, and immunogenicity.
Methods
We performed a single-center prospective observational cohort study following an elective switch from Remicade® to CT-P13 in IBD patients.
Results
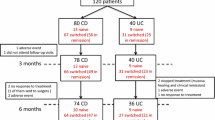
Eighty-three patients were included (57 Crohn’s disease, 24 ulcerative colitis, and 2 IBD unclassified), and 68 patients completed one-year follow-up. Disease activity (Harvey–Bradshaw Index and Simple Clinical Colitis Activity Index) as well as inflammatory markers (CRP, fecal calprotectin) did not change significantly during the 1-year follow-up. In total, 7 out of 83 patients (8%) demonstrated detectable antidrug antibodies during follow-up, and 5 out of 7 antidrug antibody titers were already detectable at baseline prior to switching. Six patients (7%) discontinued CT-P13 due to adverse events.
Conclusions
Following a switch from Remicade® to CT-P13, 82% of IBD patients continued treatment through 1 year. Disease activity scores and inflammatory markers remained unchanged during follow-up, and no CT-P13-related serious adverse events occurred. These 1-year data suggest that switching to CT-P13 in Remicade®-treated IBD patients is safe and feasible.
Similar content being viewed by others
Introduction
The tumor necrosis factor-α-targeting (anti-TNF-α) monoclonal antibody infliximab is a biological agent which is highly effective for induction and maintenance of remission in patients with inflammatory bowel disease (IBD) [1]. Implementation of the EMA- and FDA-approved infliximab biosimilar CT-P13 is expected to result in significant cost savings for the treatment of IBD patients [2, 3]. Infliximab naïve IBD patients frequently start CT-P13 in current daily practice but the switch from originator Remicade® to biosimilar CT-P13 is less common since long-term clinical data and experience are limited [4]. We recently reported the 4-month results from a prospective cohort of IBD patients who switched from Remicade® to CT-P13 [5]. Here, we report the 12-month results on efficacy, safety, pharmacokinetics, and immunogenicity.
Materials and Methods
We performed a prospective observational cohort study in a tertiary IBD referral center in Nijmegen, the Netherlands. All Remicade®-treated IBD patients switched to CT-P13 in May–June 2015, regardless of disease activity [5]. Patients continued treatment with the same infliximab dosing and infusion intervals, although dose optimization and concomitant IBD therapies were allowed. IBD characteristics were collected at baseline, including the age at IBD diagnosis, Montreal classification, and both previous and current exposure to IBD therapies. Primary endpoint was change in disease activity scores at week 52 compared to week 0 as measured by Harvey–Bradshaw Index (HBI) for Crohn’s disease (CD) and Simple Clinical Colitis Activity Index (SCCAI) for ulcerative colitis (UC) and IBD unclassified (IBD-U). Secondary endpoints included C-reactive protein (CRP), fecal calprotectin (FCP), infliximab trough levels (TL), antidrug antibodies to infliximab (ADA), and drug survival. Furthermore, we defined subgroups to compare disease activity change and drug survival between patients who were previously treated with infliximab versus who never received infliximab prior to the current Remicade episode, patients with prior anti-TNF versus never treated with anti-TNF, and patients with concomitant immunosuppressive therapy at baseline versus monotherapy infliximab. Infliximab TL were measured by a validated enzyme-linked immunosorbent assay from Sanquin Biologics Laboratory (Amsterdam, the Netherlands) [6]. A validated radioimmunoassay was used to measure the free fraction of serum ADA to infliximab, in antibody units (AU) per milliliter [7]. We first demonstrated that both assays functioned equally well for both Remicade® and CT-P13, as previously described [5]. Clinical remission was defined as HBI ≤ 4 and SCCAI ≤ 3 [8, 9]. All adverse events (AEs) and reasons for discontinuation were documented during follow-up. Requirement for written informed consent was waived [Medical Ethics Review Committee (2015-#1922)]. Results are reported as median (range minimum–maximum). We analyzed differences between week 0 versus week 52 with the Wilcoxon matched-pair signed rank test in case of skewed continuous variables. Differences between subgroups were analyzed with Chi-square for dichotomous variables and Mann–Whitney U for skewed continuous variables. A p value <0.05 was considered statistically significant. We performed an intention to treat analysis, and the “latest observation carried forward” method was used to record data from patients who discontinued CT-P13. Other missing data were excluded from analyses, and missings were considered at random.
Results
Patients
We included 83 IBD patients on Remicade® who switched to CT-P13 (57 CD, 24 UC, 2 IBD-U) (Table 1). One additional patient declined switching and was excluded. Men represented 34% of the cohort. The median age at inclusion was 36 years (range 18–79 years), and the median age at time of IBD diagnosis was 25 years (range 8–65). Median duration of ongoing Remicade® treatment at start of the study was 25 months (range 1–168).
Disease Activity
Median change in disease activity was 0 points for both CD [HBI range −23 to +15] and UC [SCCAI range −4 to +4] (Fig. 1). Clinical remission rates were 53/83 (64%) at baseline and 61/83 patients (73%) at week 52. Inflammatory biomarkers did not change during the observational period. The median level of CRP was 1.0 [range 1–42] at week 0 and 2.0 [1–56] at week 52 [p = 0.343, n = 83]. FCP was 83 [range 5–1404] at week 0 and 56 [5–957] at week 52 [p = 0.195, n = 38]. Furthermore, the change in disease activity outcomes during follow-up—including HBI, SCCAI, FCP, and CRP—was not significantly different between the analyzed subgroups (infliximab-experienced vs naive, prior anti-TNF treatment vs first anti-TNF agent, concomitant immunosuppressive therapy vs monotherapy, results not shown).
Change in disease activity scores during follow-up. Absolute change in disease activity scores at week 52 relative to week 0, after switching from Remicade® to CT-P13 in inflammatory bowel disease patients. CD Crohn’s disease, UC ulcerative colitis, HBI Harvey–Bradshaw Index, SCCAI Simple Clinical Colitis Activity Index
Pharmacokinetics and Immunogenicity
Infliximab TL remained unaffected in the one-year observational study. At week 0 median TL were 3.6 ng/ml [range 0.0–40.0], while at week 52 median TL were 3.7 ng/ml [range 0.0–17.0; p = 0.559, n = 82]. These TL included measurements during the induction phase for some patients. CT-P13 dose was intensified in 16/83 patients (19%) while reduced in 7/83 patients (8%) during follow-up, at the discretion of the treating physician. The proportion of patients with TL within the therapeutic range (3.0–7.0 ng/ml) increased from 39% (week 0) to 45% (week 52). Seven patients had detectable ADA (>12.0 AU/ml) as given in Table 2. Two of seven patients developed new ADA during follow-up.
Safety and Drug Survival
Sixty-eight patients completed one-year follow-up (Fig. 2). In total, 15 of 83 patients (18%) discontinued CT-P13 during follow-up (Table 3). The previously mentioned subgroups did not differ in discontinuation rates. In total, 39 patients (47%) reported AEs, leading in six patients (7%) to discontinuation of therapy due to skin rash (n = 2) and arthralgia (n = 4). Of the latter group, one patient had progressive arthralgia after the second infusion with CT-P13 which coincided with high ADA titers. More than 5 months after discontinuation of CT-P13 a woman died of subarachnoidal hemorrhage at the age of 80. Three serious adverse events occurred during the course of this study (4%), concerning hospital admission for intestinal obstruction, retrostomal torsion, and intra-abdominal adhesions. The latter three patients could all continue CT-P13, and no life-threatening events occurred.
Discussion
Long-term data on switching to the biosimilar CT-P13 are needed in order to provide physicians guidance in daily clinical practice [10, 11]. Although confidence about biosimilar use is increasing, immunogenicity is the main concern of IBD specialists [12]. Our study suggests that the switch from Remicade® to CT-P13 can be done safely in daily clinical IBD practice as we saw no significant changes in disease activity after one-year follow-up. Fifteen out of 83 patients discontinued CT-P13, including six patients who discontinued CT-P13 due to adverse events.
Disease activity did not change significantly during follow-up, in line with other prospective observational switch cohorts. In Oslo, 143 IBD patients showed no significant change in disease activity 6 months after switching to CT-P13 [13]. A prospective cohort study from Spain described 70/81 (86%) IBD patients who maintained remission after switching [14]. And in 39 pediatric IBD patients laboratory results remained the same 16 weeks after switching [15]. In addition, the randomized controlled NOR-SWITCH study recently showed similar disease activity in patients who switched from Remicade® to CT-P13 across all adult indications [16]. There is one caveat in this NOR-SWITCH study as disease worsening after switching occurred more frequently in CD patients. It should be pointed out that this study was not powered to draw firm conclusions in the subgroup of CD patients.
Fifteen out of 83 patients discontinued CT-P13, including 11/83 (13%) for reasons of loss of response or adverse events. This is in line with historic real-life Remicade® cohorts. The previously reported Leuven cohort included 614 Remicade®-treated CD patients with a follow-up of 4.6 years (IQR 2.3–6.9). The median annual dropout rate due to loss of response or adverse events was 7.1–10.7% [17]. The discontinuation rate for a retrospective cohort of 182 CD patients treated with scheduled Remicade® maintenance therapy at the Mayo clinic was 12, 29 and 51% after 1, 2 and 5 years, respectively [18]. Drug persistence in a prospective switch study was compared to a retrospective Remicade cohort and showed highly similar survival curves over a period of eight infusions [19].
Six patients (7%) had to discontinue CT-P13 as a result of adverse events, none of them serious. Five months after discontinuation, one 80-year-old patient died of the consequences of subarachnoidal hemorrhage from a cerebral aneurysm while using dipyridamole, which we assume is unrelated to CT-P13. The Leuven cohort reports long-term safety of infliximab in 734 IBD patients during a median follow-up of 58 months. Discontinuation due to adverse events occurred in approximately 21%, including serious infections in 6.5% and acute or delayed infusion reactions in 9.5% [20].
Our one-year data on relevant clinical outcomes in a prospective study will aid in clinical decision making regarding switching to CT-P13. There is a paucity of data regarding long-term follow-up after switching. Our study comes with limitations, such as the absence of a control group that allowed patients to continue Remicade®. In addition, the cohort was heterogeneous in terms of disease activity and infusion schedule. On the other hand, this reflects real-world practice in IBD.
In conclusion, our one-year data on switching from Remicade® to biosimilar CT-P13 in a real-life cohort of IBD patients demonstrated no significant impact on clinical outcomes that included disease activity, safety, drug survival, and pharmacokinetics. These outcomes support feasibility for switching to CT-P13.
Abbreviations
- IBD:
-
Inflammatory bowel disease
- CD:
-
Crohn’s disease
- UC:
-
Ulcerative colitis
- IBD-U:
-
IBD unclassified
- HBI:
-
Harvey–Bradshaw Index
- SCCAI:
-
Simple Clinical Colitis Activity Index
- CRP:
-
C-reactive protein
- FCP:
-
Fecal calprotectin
- TL:
-
Infliximab trough levels
- ADA:
-
Antidrug antibodies to infliximab
- EMA:
-
European Medicines Agency
- FDA:
-
Food and Drug Administration
- TNF:
-
Tumor necrosis factor
- AE:
-
Adverse event
- SAE:
-
Serious adverse event
- NA:
-
Not applicable
- IFX:
-
Infliximab
- 5-ASA:
-
5-Aminosalicylic acid
- MTX:
-
Methotrexate
- 6-TG:
-
6-Thioguanine
- AZA:
-
Azathioprine
References
Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549.
Brodszky V, Rencz F, Pentek M, Baji P, Lakatos PL, Gulacsi L. A budget impact model for biosimilar infliximab in Crohn’s disease in Bulgaria, the Czech Republic, Hungary, Poland, Romania, and Slovakia. Expert Rev Pharmacoecon Outcomes Res. 2016;16:119–125.
Mulcahy AW, Predmore Z, Mattke S. The Cost Savings Potential of Biosimilar Drugs in the United States. City: RAND Corporation. Available at: http://www.rand.org/pubs/perspectives/PE127.html;2014.
Danese S, Fiorino G, Raine T, et al. ECCO position statement on the use of biosimilars for inflammatory bowel disease—an update. J Crohn’s Colitis. 2017;11:26–34.
Smits LJ, Derikx LA, de Jong DJ, et al. Clinical outcomes following a switch from Remicade® to the biosimilar CT-P13 in inflammatory bowel disease patients: a prospective observational cohort study. J Crohn’s Colitis. 2016;10:1287–1293.
Vande Casteele N, Buurman DJ, Sturkenboom MG, et al. Detection of infliximab levels and anti-infliximab antibodies: a comparison of three different assays. Aliment Pharmacol Ther. 2012;36:765–771.
Wolbink GJ, Vis M, Lems W, et al. Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:711–715.
Vermeire S, Schreiber S, Sandborn WJ, Dubois C, Rutgeerts P. Correlation between the Crohn’s disease activity and Harvey–Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol. 2010;8:357–363.
Falvey JD, Hoskin T, Meijer B, et al. Disease activity assessment in IBD: clinical indices and biomarkers fail to predict endoscopic remission. Inflamm Bowel Dis. 2015;21:824–831.
Martelli L, Peyrin-Biroulet L. Efficacy, safety and immunogenicity of biosimilars in inflammatory bowel diseases: a systematic review. Curr Med Chem. 2016 [Epub ahead of print].
Ben-Horin S, Vande Casteele N, Schreiber S, Lakatos PL. Biosimilars in inflammatory bowel disease: facts and fears of extrapolation. Clin Gastroenterol Hepatol. 2016;14:1685–1696.
Danese S, Fiorino G, Michetti P. Changes in biosimilar knowledge among European Crohn’s Colitis Organization [ECCO] members: an updated survey. J Crohn’s Colitis. 2016;10:1362–1365.
Buer LC, Moum BA, Cvancarova M, Warren DJ, Medhus AW, Hoivik ML. Switching from Remicade® to Remsima® is well tolerated and feasible: a prospective, open-label study. J Crohn’s Colitis. 2017;11:297–304.
Arguelles-Arias F, Guerra Veloz MF, Perea Amarillo R, et al. Effectiveness and safety of CT-P13 (biosimilar infliximab) in patients with inflammatory bowel disease in real life at 6 months. Dig Dis Sci. 2017. doi:10.1007/s10620-017-4511-4.
Sieczkowska J, Jarzebicka D, Banaszkiewicz A, et al. Switching between infliximab originator and biosimilar in paediatric patients with inflammatory bowel disease. Preliminary observations. J Crohn’s Colitis. 2016;10:127–132.
Jørgensen KK, Olsen IC, Goll GL, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017;389:2304–2316.
Schnitzler F, Fidder H, Ferrante M, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut. 2009;58:492–500.
Seminerio JL, Loftus EV Jr, Colombel JF, Thapa P, Sandborn WJ. Infliximab for Crohn’s disease: the first 500 patients followed up through 2009. Dig Dis Sci. 2013;58:797–806.
Razanskaite V, Bettey M, Downey L, et al. Biosimilar Infliximab in inflammatory bowel disease: outcomes of a managed switching programme. J Crohn’s Colitis. 2017;11:690–696.
Fidder H, Schnitzler F, Ferrante M, et al. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut. 2009;58:501–508.
Acknowledgments
We thank Dr. Wietske Kievit, Radboudumc, for her invaluable help in designing the study.
Author’s contribution
LD, FH, JD, and LS all contributed to the design of the study. LS collected and analyzed the data. FH, AG, and LS drafted the manuscript. LD and JD critically revised the manuscript for important intellectual content. RB, DJ, and AE provided data and critically revised the manuscript. All authors approved the submitted manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DJ received consulting fees from Synthon Pharma, Abbvie, and MSD, and travel fees from Falk Pharma, Takeda, Abbvie, MSD, Ferring, Vifor Pharma, and Cablon Medical. FH has served on advisory boards of MSD, Takeda, Celltrion, and Dr. Falk and served as a consultant for Celgene. JD has served on advisory boards of Janssen, AbbVie, BMS, Gilead, and served as a consultant for Gilead. His department receives research funding from Gilead, Abbvie, Ipsen, and Novartis.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Smits, L.J.T., Grelack, A., Derikx, L.A.A.P. et al. Long-Term Clinical Outcomes After Switching from Remicade® to Biosimilar CT-P13 in Inflammatory Bowel Disease. Dig Dis Sci 62, 3117–3122 (2017). https://doi.org/10.1007/s10620-017-4661-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4661-4