Abstract
In 2011, the European League Against Rheumatism (EULAR) published recommendations regarding the vaccination of children with rheumatic diseases. These recommendations were based on a systematic literature review published in that same year. Since then, the evidence body on this topic has grown substantially. This review provides an update of the systematic literature study of 2011, summarizing all the available evidence on the safety and immunogenicity of vaccination in paediatric patients with rheumatic diseases. The current search yielded 21 articles, in addition to the 27 articles described in the 2011 review. In general, vaccines are immunogenic and safe in this patient population. The effect of immunosuppressive drugs on the immunogenicity of vaccines was not detrimental for glucocorticosteroids and methotrexate. Biologicals could accelerate a waning of antibody levels over time, although most patients were initially protected adequately. Overall, persistence of immunological memory may be reduced in children with rheumatic diseases, which shows the need for (booster) vaccination. This update of the 2011 systematic literature review strengthens the evidence base for the EULAR recommendations, and it must be concluded that vaccinations in patients with rheumatic diseases should be advocated.
Similar content being viewed by others
Introduction
Children with paediatric rheumatic diseases (pedRD) have an increased risk of infection, which contributes to the mortality and morbidity of their disease [1–3]. Effective and safe vaccination is key in prevention of numerous of these infections.
Assessing efficacy of a vaccine in patients with pedRDs is challenging. The ideal measure of efficacy, infection rates, is usually not studied as a primary endpoint because this requires large sample sizes. Surrogate measures such as immunogenicity are commonly used instead. Immunogenicity refers to the immune response induced by vaccination. This is usually measured by vaccine-specific geometric mean antibody titers (GMT) or concentrations (GMC), seroconversion rates and/or seroprotection rates. The measure for immunogenicity differs per vaccine, as the relation between the humoral and/or cellular immune response and protection differs per pathogen [4–6]. Immunogenicity of a vaccine in patients with rheumatic diseases can differ from the healthy population, due to the disease or its immunosuppressive treatment.
Besides short-term vaccine-induced immune responses, persistence of protective immunologic memory after vaccination is essential in preventing infections [7, 8]. As this persistence goes beyond follow-up of most studies in rheumatic diseases, long-term effectiveness of most vaccines is unknown.
The safety of vaccines in pedRD can be addressed on different levels: adverse event rate in comparison to healthy controls, increased disease activity induced by vaccination and unintentional infections induced by live-attenuated pathogens in vaccines (especially in patients on high-dose immunosuppressive drugs). Another issue of vaccine safety is whether vaccines or their constituents can actually cause autoimmune disease (AID), which will be addressed briefly.
Over the years, awareness of infection prevention by vaccination in rheumatic diseases has increased. In 2011, a EULAR task force published evidence-based recommendations regarding vaccination of adults and children with rheumatic diseases. A year later, the Brazilian Society of Rheumatology published vaccination recommendations for patients with rheumatoid arthritis (RA) [9, 10, 11••].
According to these recommendations, non-live vaccines are generally adequately immunogenic and safe. Live-attenuated vaccines can be administered to patients with pedRD, unless they are on high-dose immunosuppressive drugs or biologicals. In these cases, evidence on safety is scarce but reassuring. Therefore, live-attenuated booster vaccinations can be considered on individual basis.
Not all vaccines have been studied in pedRD patients, most studies do not take persistence of immunological memory into account, and studies were often underpowered and uncontrolled to assess safety. Consequently, concerns regarding efficacy and safety of vaccines remain. Providing a periodical overview of new evidence, as advised in the EULAR recommendations, is necessary to assure effective and safe vaccination in this vulnerable group.
In this review, we provide an update of the evidence on vaccination of pedRD patients published since the EULAR recommendations in 2011 [12••]. The influence of immunosuppressive drugs and biologicals on immunogenicity and safety of non-live composite as well as live-attenuated vaccines will be addressed. Additionally, we describe the use of adjuvants and their possible association with adverse events (AE).
A systematic literature review was performed in July 2014, following the methodology described earlier [12••]. Since the first systematic literature review describing 27 papers, 21 additional eligible articles on vaccination of patients with pedRD have been published (Fig. 1). A large portion (n = 10) of the new studies investigated the immunogenicity of the seasonal influenza or H1N1 vaccine. The pedRD studied most (n = 13) was juvenile idiopathic arthritis (JIA). Eleven new studies described the influence of biologicals on immunogenicity of the vaccine, adding to the five studies described earlier. Three additional articles were found which studied live-attenuated vaccines to the six articles included in 2011. Two new studies were randomized controlled trials (RCTs). This is the study design of choice when assessing the effect of vaccination on disease activity in pedRD [13••, 14••].
The search strategy for the systematic literature review [12••]. The disease search encompassed articles on vaccination in patients with paediatric autoinflammatory or rheumatic diseases, and the medication search encompassed articles on vaccination and immunosuppressive drugs
Vaccine Immunogenicity in Paediatric Patients With Rheumatic Diseases
Most studies assessed short-term vaccine-induced immunogenicity. Five studies measured antibody levels up to 12 months post-vaccination [13••, 14••, 15–17]. Another five studies evaluated antibody persistence several years after vaccination [18–22]. Although some studies studied actual occurrence of infections such as herpes zoster (HZ) or influenza, they were underpowered to assess these outcomes reliably [13••, 23, 24].
Below we summarize and discuss all available evidence found in the previous and current systematic literature [12••].
Immunogenicity in Relation to Immunosuppressive Drugs
Glucocorticosteroids
Eleven articles included 401 patients using glucocorticosteroids (GC). Of them, the majority used a low dose (<20 mg/day) [17, 18, 25–27, 29–32, 43] (Table 1). Patients who use GC may show lower seroconversion rates or GMT, but they generally still reach protective antibody titers. A high dose of GC or concomitant use of other immunosuppressive drugs was associated with lower, yet still protective, responses in several studies. No effect of GC on the persistence of several vaccine-specific antibodies (mumps, measles and rubella vaccine (MMR), tetanus-diphtheria vaccine (TD)) could be found in one study [18]. These findings show that there is no general detrimental effect of low-dose GC on immunogenicity or established antibody levels.
Methotrexate
Eight studies including 420 patients on methotrexate (MTX) were available [18, 20, 26, 27, 33–36] (Table 1). No effect of MTX was found on short-term immunogenicity of vaccines or on the persistence of antibodies over time [18, 22].
Biologicals
A total of 296 patients using biologicals were included in 15 studies [13••, 14••, 21–24, 28, 34–41] (Table 1). The biologicals most frequently studied were tumour necrosis factor (TNF)α blockers. The majority of patients reached protective antibody concentrations after vaccination, but in the majority of studies the actual antibody concentrations of patients using biologicals were lower than of patients who did not. Additionally, the antibody levels declined more rapidly over time in patients using biologicals [22, 41]. A lower initial GMT and a more rapid decline in antibody levels will lead to a quicker decrease in seroprotection rate in these patients. Monitoring GMTs and additional booster vaccinations should be considered in order to ensure protection in these patients. Another option is to administer specific vaccines prior to start of biological therapy.
Immunogenicity of Non-live Composite Vaccines
Human Papillomavirus Vaccine
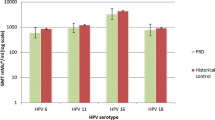
Currently, there are two human papilloma virus (HPV) vaccines: the quadrivalent (qHPV) vaccine (against HPV 6, 11, 16 and 18) and the bivalent (bHPV) vaccine (against HPV 16 and 18). At the time the EULAR recommendations were published, no publications regarding the immunogenicity or safety of either HPV vaccine in pedRD were available. The recommendation was based on preliminary data [10]. Since then, three articles assessing the immunogenicity and safety of the HPV vaccine in pedRD have been published [15, 16, 52] (Table 2).
The immunogenicity of the bivalent vaccine in 63 JIA patients was compared to 48 healthy controls, showing that all participants were seropositive up to 12 months after vaccination. GMCs were lower in patients than in controls, but no statistical significant difference in GMC over time was found [15].
Adequate immunogenicity of HPV vaccination is of specific interest in patients with systemic lupus erythematosus (SLE), as these patients have a high risk of persistent HPV infections [62–64]. Two studies included a total of 33 juvenile SLE (jSLE) patients, one including a control group of 49 healthy children. Both studies showed that the majority of patients seroconverted [16, 52]. Interestingly, the pilot study including six jSLE patients reported lower antibody concentrations in patients than in controls [16]. A study in 39 adult SLE patients also showed lower GMCs in patients than in healthy controls [65]. Based on these results, the long-term protection against HPV infections in SLE patients is unclear. Larger, controlled studies in jSLE patients are necessary to assess the immunogenicity of the HPV vaccine in this group.
Seasonal Influenza Virus and H1N1 Vaccine
Fifteen articles described the immunogenicity of seasonal influenza and H1N1 vaccines in pedRD (Table 2). They included 899 pedRD patients [23, 24, 27–29, 31, 32, 35, 36, 41–43, 56, 58]. One study group described a similar study population in three articles [31, 36, 58]. The three overlapping studies are described separately in Table 2, as it was impossible to disentangle the data.
Although antibody concentrations in patients were lower, seroprotection against influenza was similar in patients and controls. Two studies including 209 jSLE patients showed that this specific group has significantly lower seroconversion rates, seroprotection rates and GMT than healthy controls. Lower responses were not related to medications used and were possibly associated with a higher SLE disease activity index [32].
Two studies assessed the incidence of respiratory infections and influenza-like illness. Due to their small study population, no definite conclusions could be drawn regarding vaccine efficacy [23, 24].
Hepatitis A and hepatitis B Vaccine
One new study on the immunogenicity of the hepatitis A virus (HAV) vaccine was found [54]. Two studies including 57 patients showed an adequate immunogenicity in patients not using anti-TNFα treatment [40, 53]. One study in twelve children with inflammatory bowel syndrome using anti-TNFα treatment showed an adequate seroconversion rate of 92 % [54] (Table 2).
Hepatitis B virus (HBV) vaccines were studied in 245 patients [19, 21, 26, 30, 53] (Table 2). After vaccination, the majority of the patients and all of the healthy controls had protective antibody levels. However, the persistence of protective immunity against HBV may be lower in pedRD patients [19, 21]. The reduced proportion of patients that is protected directly after vaccination, together with the low percentage of protected patients several years after vaccination, illustrates that the humoral response after HBV vaccination should be checked and that patients could benefit from a booster vaccine.
Meningococcal Vaccine
One new study was found, in addition to the previously described study on the Neisseria meningitides C (NeisVac-C) vaccine, which was safe and immunogenic in 234 JIA patients [22, 59] (Table 2). In this study, MenC-IgG levels were assessed over time in 127 patients with JIA and 1527 healthy controls [22]. IgG levels decreased over time, with a faster decline in younger patients. Four years after vaccination, MenC-IgG levels in JIA patients were similar to those in healthy controls. Patients who had started biologicals showed an accelerated decline in antibody levels.
Pneumococcal Vaccines
No new studies were found on pneumococcal vaccines. In the previous review, one study was found (Table 2). It showed that JIA patients had a similar response and seroprotection rate to the 7-valent pneumococcal vaccine (PCV7) as healthy controls when using MTX or cyclosporine, either with or without concomitant GC use. Patients using anti-TNFα were all seroprotected, but had significantly lower antibody concentrations [39].
Tetanus-Diphtheria Vaccine
One new study was added to the evidence from four studies previously found on immunogenicity of the tetanus toxoid (TT) or tetanus-diphtheria (TD) vaccine [20, 55, 60, 61] (Table 2). The previously found studies (95 patients, 125 controls) showed comparable antibody levels to controls. Two studies in 430 pedRD patients assessed persistence of these antibodies over time. Both showed lower concentrations and seroprotection rates than in a comparable healthy control group after 7–16 years of follow-up [18, 20].
Other Non-live Composite Vaccines
No articles were found containing information on Haemophilus influenza type B (HiB) vaccines, pertussis vaccines or inactivated poliovirus vaccines. No information was found on vaccines indicated for endemic areas such as vaccines against typhoid fever, tick-borne encephalitis (FSME), rabies, Japanese encephalitis or cholera.
Live-Attenuated Vaccines
Measles, Mumps and Rubella Vaccine
In the previous systematic literature review, only one study in ten JIA patients assessed short-term immunogenicity of the MMR booster. It showed a cellular and humoral immune response comparable to healthy controls [34]. One additional article on the immunogenicity of the MMR booster vaccination was published. This RCT showed that all 68 vaccinated patients displayed a significant increase in MMR antibody concentrations. All patients were seroprotected against MMR at 12 months after vaccination [14••] (Table 2).
Two studies reported on the persistence of antibodies several years after MMR vaccination in patients with JIA or jSLE. Both studies found similar levels of protective antibodies against measles in patients and controls 7–16 years after two MMR doses in the first year of life [20] and in all age groups (1–19 years) after one or two MMR doses. Protective antibody levels against mumps and rubella up to 10 years after MMR booster vaccination were significantly lower in JIA patients than in controls. Patients had an odds ratio of 0.4 to be seroprotected against mumps or rubella compared to controls (adjusted for age and number of vaccinations) [18] (Table 2).
Varicella Zoster Vaccine
In the previous review, two studies regarding the varicella zoster virus (VZV) vaccine were found. A controlled study including 25 pedRD patients and 18 healthy controls found a lower response rate in patients than in controls after vaccination. Of the eight patients who reported having contact with a VZV-infected individual, two (both non-responders), developed chickenpox [17]. A case series reported six IBD patients having positive immunity after vaccination [38]. One new study, an RCT including 54 jSLE patients of whom 28 were vaccinated, has been found in the new search. Only patients who used either cyclosporine, azathioprine, methotrexate and/or GC up to 20 mg/day were included in this study. All participants had protective antibody levels against VZV at baseline. Patients had a similar increase in GMT as the healthy control group, and all had a significant increase in antibody levels compared to baseline. Over 35.6 months of follow-up after vaccination, four cases of HZ were reported in the unvaccinated group whereas no HZ occurred in the vaccinated group [13••] (Table 2).
In adults, two large studies illustrate the importance of effective vaccination against VZV. A meta-analysis in adults with rheumatic diseases (RD) showed that the risk of HZ infections is increased by up to 61 % in patients using biologicals compared to patients using conventional disease-modifying anti-rheumatic drugs (DMARDs) [66]. A retrospective cohort study in 7780 vaccinated and 455,761 unvaccinated adults with RD assessed vaccine efficacy (incidence of HZ infections >42 days after vaccination). They showed a significantly lower hazard ratio for HZ infections (HR 0.61, 95 % CI 0.59–0.75) in vaccinated patients up to 2 years of follow-up [67•].
Bacillus Calmette-Guérin Vaccine
No new evidence was found on the immunogenicity of the Bacillus Calmette-Guérin (BCG) vaccine in pedRD patients [27, 44–49] (Table 2). In the 2011 review, seven papers were described including 15,810 Kawasaki disease (KD) patients and 115 JIA patients. It is suggested that JIA patients have lower protection rates after vaccination, due to their lower tuberculin skin test induration size. The remaining articles did not assess immunogenicity. As the vaccine causes local inflammation at the BCG vaccination site in up to 50 % of KD patients, withholding the BCG vaccine in active KD was advised [11••].
Yellow Fever Vaccine
No studies were found on the immunogenicity of the yellow fever (YF) vaccine in children with pedRD, but it has been studied in 91 adult patients with RD. In these patients, the vaccine had good immunogenicity. The responses were reduced in the 26 patients who used anti-TNFα therapy [71]. The EULAR stated that booster vaccinations against YF can be considered in patients on MTX less than 15 mg/m2/week or low-dose GC [12••].
Vaccine Safety in Paediatric Patients With Rheumatic Diseases
Adverse Events and Serious Adverse Events
Adverse events (AE) and serious adverse events (SAE) were registered in the majority of the studies. None found relevant differences in AE between patients and controls and no SAE related to vaccination were reported.
Preliminary data on thromboembolic events after qHPV vaccination resulted in the EULAR recommendation to be vigilant for these complications [11••]. Based on current literature, this seems unnecessary as a large cohort study in 997,585 healthy girls, of whom 296,826 received at least one dose of the qHPV vaccine, showed no evidence of an association between qHPV vaccination and venous thromboembolic adverse events [68•].
Disease Activity Induced by Vaccination
As most pedRD are very unpredictable in disease activity and flares, the only reliable method to assess the effect of vaccination on disease activity is an RCT study design. This way, results are corrected for the relapsing-remitting course of the disease. Two RCTs assessed the effect of the live-attenuated MMR booster vaccination on JIA, respectively, the VZV vaccination on jSLE disease activity. Both studies reported similar disease activity and flare rates in vaccinated patients and disease-controls [13••, 14••]. Some non-randomized studies included an unvaccinated control group of patients. These studies suffered from selection bias since the unvaccinated group had lower disease activity at baseline [24] or the control group was not described at all [30].
Most studies assessing disease activity used patients as their own control. These studies reported a stable disease activity over time or similar flare rates before and after vaccination (Table 2). One case report described a systemic onset JIA (soJIA) patient on anti-IL6 who received two seasonal influenza vaccines and had a disease flare after both vaccinations [57]. In contrast, a study in 27 soJIA patients using anti-IL6 receiving a seasonal influenza vaccine did not show any exacerbations [42]. In summary, studies do not show an increase in disease activity after vaccinations in the patient population as a whole. This is unequivocally shown by the RCTs with live-attenuated vaccines. Of course, the theoretical possibility remains that individual patients are susceptible for aggravation of disease after vaccination due to disproportionate immune responses. However, this theoretic possibility should not result in refraining from current immunization practice, because the benefits of infection prevention significantly outweigh the small risk of a disease flare in patients.
Induction of Infections With Attenuated Pathogens
The possibility of the induction of infections with an attenuated pathogens after live-attenuated vaccines is a matter of concern especially in patients on high-dose immunosuppressive drugs or biologicals. Only the RCT assessing the safety of the live-attenuated MMR vaccine included patients using biologicals (n = 9). They did not have infections with the live-attenuated pathogens [14••]. A cohort study including 25 VZV-vaccinated pedRD patients showed no overt varicella episodes within 40 days after vaccination. Three patients did develop a mild, self-limiting varicella-like rash, but this was not accompanied by any other symptoms [17]. A study in 7780 adult patients with RD reported 11 HZ cases within 42 days after vaccination, suggestive for vaccination-induced herpes infections [67•]. Reassuringly, no vaccination-induced HZ infections were detected in the 633 patients on biologicals.
Information on the BCG vaccination in patients on high-dose immunosuppressive drugs or biologicals is lacking. There is a very high rate of complications in patients who are severely immunocompromised, such as SCID patients [69]. One case report described a 3-month-old infant born to a mother with Crohn’s diseases using infliximab who had a lethal vaccination-induced mycobacterial infection after BCG vaccination [70]. Based on these data, BCG vaccines should therefore not be administered to patients using biologicals or high doses of immunosuppression.
There is no information available on the safety of the live-attenuated YF vaccine in patients with pedRD, but the vaccine was safe in adult patients with RD [71].
Adjuvant Safety in Paediatric Patients With Rheumatic Diseases
Adjuvants are added to vaccines to enhance the immune response to the vaccine-antigen. Frequently used adjuvants (alum, Toll-like receptor (TLR) four ligand monophosphoryl lipid A adsorbed to alum (ASO4) and oil in water emulsions like AS03 or MF59) stimulate pattern-recognition receptors (PRRs) such as TLRs. TLRs are expressed on cells like dendritic cells, which in turn determine the magnitude and quality of the adaptive immune response [72–74]. Through these mechanisms, adjuvants could theoretically also trigger or enhance autoimmune responses in patients with established AID [74, 75].
The safety of adjuvants in rheumatic diseases has not been studied well. RCTs, in which patients with rheumatic diseases are vaccinated with adjuvanted versus unadjuvanted vaccines are lacking. In this review, we found 12 reports including 614 patients that studied an adjuvanted non-live vaccine [15, 16, 21, 26, 30, 35, 39, 41, 52–54, 59]. Seven reports including 499 patients studied a non-adjuvanted non-live vaccine [24, 25, 31, 32, 36, 56, 58]. It was unclear whether the vaccine was adjuvanted in eight reports [19, 22, 23, 40, 42, 55, 57, 61]. No marked increase in disease activity was seen in the patients receiving an adjuvanted vaccine compared to the patients who received an unadjuvanted dead composite vaccine. Based on these results, it does not seem likely that adjuvants cause a significant deterioration of disease activity in paediatric patients with rheumatic diseases.
In theory, adjuvants could also be part of the causal pathway in the onset of AID. Anecdotal evidence for this relation has been published [75, 76], and a syndrome of shared clinical symptoms thought to be caused by adjuvants, the autoimmune/inflammatory syndrome induced by adjuvants (ASIA), has been postulated [76]. Recently, a large epidemiological study applying the ASIA diagnostic criteria to a population vaccinated with the HPV vaccine has been performed. A total of 57 million administrated doses were reported and 26,508 self-reports on AEs were found. Of these, 3932 cases could be classified as ASIA, defined by flu-like symptoms such as fever, myalgia, arthralgia or arthritis. In 2634 cases, a probable or possible association with HPV vaccination could be made. However, no mention was made about the duration of the complaints, and a comparison of the frequency of similar complaints in an unvaccinated population was not made [77].
Several large studies have not found any association between vaccination and AID. A large register-based cohort study including 997,585 girls aged 10–17 years, among whom 296,826 received a total of 696,420 qHPV vaccine doses, no association between exposure to the qHPV vaccine and autoimmune adverse events was found [68•]. Also, analysis of over 68,000 participants who received AS04-adjuvanted vaccines or served as controls demonstrated a low rate of autoimmune disorders, without evidence of an increase in relative risk associated with AS04-adjuvanted vaccines [78]. Finally, a review of reported adverse reactions after the pandemic influenza A/H1N1 vaccine using EudraVigilance data and literature did not reveal a difference between autoimmune phenomena after adjuvanted or non-adjuvanted A/H1N1 vaccines [79]. Thus, the possible relation between vaccine adjuvants and the induction of autoimmune rheumatic diseases is thus far not substantiated.
Discussion
The current systematic literature review found 21 articles on vaccinations in pedRD published since the last systematic literature from 2011. The new evidence, selected using the same criteria as the first review, was added to the 27 previously described studies [12••].
Vaccines are generally immunogenic in patients with pedRD. The validity of available evidence for the effect of immunosuppressive drugs on immunogenicity was moderate or low. To accurately assess the effect of a drug on the immunogenicity of a vaccine, patients using these drugs need to be compared to patients who are drug-free or are using a minimal amount of immunosuppression. Few studies included such a comparison, so only indirect conclusions on the effects of GC, MTX and biologicals could be drawn. GC, predominantly studied in a low dose (<20 mg/day), and MTX do not have detrimental effects on the immune response. More evidence has become available on the effect of biologicals, especially anti-TNFα treatment, on (long-term) immunogenicity of vaccines. Although seroprotection rates are usually adequate, antibody concentrations are lower in patients using biologicals.
To ensure long-term protection against vaccine-preventable infections, protective antibody levels should be persistent. Persistence of protective antibody levels is lower in pedRD patients than healthy controls for some, but not all, pathogens [18, 22]. Biological use seems to accelerate the natural decline of antibody levels, besides lowering the vaccine-induced antibody concentrations. Therefore, regular assessment of antibody levels and subsequent administration of booster vaccines in these patients is important to ensure long-term protection. Studies in healthy individuals suggest that circulating antibody levels alone may not be predictive of long-term protection, as cellular immunity can persist independent of antibody levels [80, 81]. Assessment of cellular memory in vaccinated pedRD patients could help to study long-term protection against vaccine-preventable diseases.
Evidence on the efficacy (i.e. infection prevention) of vaccines in pedRD is still lacking. The studies that measured infection rates in vaccinated and unvaccinated patients were underpowered for definite conclusions on efficacy.
Regarding safety, vaccinations do not cause serious adverse events. Disease activity is not influenced by vaccination in the majority of the patients, now unequivocally shown for the MMR vaccination in JIA patients and the VZV vaccination in jSLE patients. No evidence has been found that adjuvants cause a higher disease activity in pedRD.
No vaccine-induced infections with live-attenuated viruses were reported in vaccinated JIA or jSLE patients after the MMR and VZV booster vaccination, respectively. Therefore, it seems that these booster vaccinations can be administered to pedRD patients, even in patients using biologicals. BCG vaccinations should not be administered to patients on high-dose immunosuppressive drugs or biologicals due to lack of safety data. Larger, controlled studies are necessary to study rare serious adverse events, especially in patients on high-dose immunosuppressive drugs or biologicals.
Much information on vaccination in pedRD has been gained in the time since the initial systematic review. For some vaccines, high-quality studies have been performed that show that they are generally immunogenic and safe. Additionally, the need for (booster) vaccinations in pedRD has been illustrated by the papers published on reduced persistence of immunological memory over time.
More evidence on the influence of biologicals on the immune response and safety of vaccines is required. Although we have information on the immunogenicity of many vaccines, this does remain a surrogate endpoint. The efficacy, namely a decrease in infection rates in pedRD, needs to be studied in larger cohorts.
While more information will be gathered over the coming years, we can now conclude that vaccinations in pedRD should be advocated. Paediatric rheumatologists should be pro-active in assessing protective antibody levels in pedRD patients and should, in line with the EULAR-recommendations, administer booster vaccines to children who are not adequately protected.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Fessler BJ. Infectious diseases in systemic lupus erythematosus: risk factors, management and prophylaxis. Best Pract Res Clin Rheumatol. 2002;16(2):281–91.
Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287–93.
Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275–85.
Thakur A, Pedersen LE, Jungersen G. Immune markers and correlates of protection for vaccine induced immune responses. Vaccine. 2012;30(33):4907–20.
Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055–65.
Viana PO, Ono E, Miyamoto M, Salomao R, Costa-Carvalho BT, Weckx LY, et al. Humoral and cellular immune responses to measles and tetanus: the importance of elapsed time since last exposure and the nature of the antigen. J Clin Immunol. 2010;30(4):574–82.
van der Maas NA, Mooi FR, de Greeff SC, Berbers GA, Spaendonck MA, de Melker HE. Pertussis in the Netherlands, is the current vaccination strategy sufficient to reduce disease burden in young infants? Vaccine. 2013;31(41):4541–7.
Davidkin I, Jokinen S, Broman M, Leinikki P, Peltola H. Persistence of measles, mumps, and rubella antibodies in an MMR-vaccinated cohort: a 20-year follow-up. J Infect Dis. 2008;197(7):950–6.
Brenol CV, da Mota LM, Cruz BA, Pileggi GS, Pereira IA, Rezende LS, et al. 2012 Brazilian Society of Rheumatology Consensus on vaccination of patients with rheumatoid arthritis. Rev Bras Reumatol. 2013;53(1):4–23.
van Assen S, Agmon-Levin N, Elkayam O, Cervera R, Doran MF, Dougados M, et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2011;70(3):414–22.
Heijstek MW, Ott de Bruin LM, Bijl M, Borrow R, van der Klis F, Kone-Paut I, et al. EULAR recommendations for vaccination in paediatric patients with rheumatic diseases. Ann Rheum Dis. 2011;70(10):1704–12. Presents the evidence-based EULAR recommendations on vaccination in pedRD.
Heijstek MW, Ott de Bruin LM, Borrow R, van der Klis F, Kone-Paut I, Fasth A, et al. Vaccination in paediatric patients with auto-immune rheumatic diseases: a systemic literature review for the European League against Rheumatism evidence-based recommendations. Autoimmun Rev. 2011;11(2):112–22. Presents methodology and the evidence base for the EULAR recommendations on vaccination in pedRD, and is supplemented with new evidence in this review.
Barbosa CM, Terreri MT, Rosario PO, de Moraes-Pinto MI, Silva CA, Hilario MO. Immune response and tolerability of varicella vaccine in children and adolescents with systemic lupus erythematosus previously exposed to varicella-zoster virus. Clin Exp Rheumatol. 2012;30(5):791–8. One of the two randomized controlled trials on vaccination in pedRD, which is the study design of choice to assess the effect of the vaccine on disease activity.
Heijstek MW, Kamphuis S, Armbrust W, Swart J, Gorter S, de Vries LD, et al. Effects of the live attenuated measles-mumps-rubella booster vaccination on disease activity in patients with juvenile idiopathic arthritis: a randomized trial. JAMA. 2013;309(23):2449–56. One of the two randomized controlled trials on vaccination in pedRD, which is the study design of choice to assess the effect of the vaccine on disease activity.
Heijstek MW, Scherpenisse M, Groot N, Tacke C, Schepp RM, Buisman AM, et al. Immunogenicity and safety of the bivalent HPV vaccine in female patients with juvenile idiopathic arthritis: a prospective controlled observational cohort study. Ann Rheum Dis. 2014;73(8):1500–7.
Heijstek MW, Scherpenisse M, Groot N, Wulffraat NM, Van Der Klis FR. Immunogenicity of the bivalent human papillomavirus vaccine in adolescents with juvenile systemic lupus erythematosus or juvenile dermatomyositis. J Rheumatol. 2013;40(9):1626–7.
Pileggi GS, de Souza CB, Ferriani VP. Safety and immunogenicity of varicella vaccine in patients with juvenile rheumatic diseases receiving methotrexate and corticosteroids. Arthritis Care Res (Hoboken). 2010;62(7):1034–9.
Heijstek MW, van Gageldonk PG, Berbers GA, Wulffraat NM. Differences in persistence of measles, mumps, rubella, diphtheria and tetanus antibodies between children with rheumatic disease and healthy controls: a retrospective cross-sectional study. Ann Rheum Dis. 2012;71(6):948–54.
Maritsi D, Vartzelis G, Soldatou A, Garoufi A, Spyridis N. Markedly decreased antibody titers against hepatitis B in previously immunised children presenting with juvenile idiopathic arthritis. Clin Exp Rheumatol. 2013;31(6):969–73.
Miyamoto M, Ono E, Barbosa C, Terreri M, Hilario M, Salomao R, et al. Vaccine antibodies and T- and B-cell interaction in juvenile systemic lupus erythematosus. Lupus. 2011;20(7):736–44.
Moses J, Alkhouri N, Shannon A, Raig K, Lopez R, Danziger-Isakov L, et al. Hepatitis B immunity and response to booster vaccination in children with inflammatory bowel disease treated with infliximab. Am J Gastroenterol. 2012;107(1):133–8.
Stoof SP, Heijstek MW, Sijssens KM, van der Klis F, Sanders EA, Teunis PF, et al. Kinetics of the long-term antibody response after meningococcal C vaccination in patients with juvenile idiopathic arthritis: a retrospective cohort study. Ann Rheum Dis. 2014;73(4):728–34.
Carvalho LM, de Paula FE, Silvestre RV, Roberti LR, Arruda E, Mello WA, et al. Prospective surveillance study of acute respiratory infections, influenza-like illness and seasonal influenza vaccine in a cohort of juvenile idiopathic arthritis patients. Pediatr Rheumatol Online J. 2013;11:10.
Toplak N, Subelj V, Kveder T, Cucnik S, Prosenc K, Trampus-Bakija A, et al. Safety and efficacy of influenza vaccination in a prospective longitudinal study of 31 children with juvenile idiopathic arthritis. Clin Exp Rheumatol. 2012;30(3):436–44.
Kanakoudi-Tsakalidou F, Trachana M, Pratsidou-Gertsi P, Tsitsami E, Kyriazopoulou-Dalaina V. Influenza vaccination in children with chronic rheumatic diseases and long-term immunosuppressive therapy. Clin Exp Rheumatol. 2001;19(5):589–94.
Kasapcopur O, Cullu F, Kamburoglu-Goksel A, Cam H, Akdenizli E, Calykan S, et al. Hepatitis B vaccination in children with juvenile idiopathic arthritis. Ann Rheum Dis. 2004;63(9):1128–30.
Kiray E, Kasapcopur O, Bas V, Kamburoglu-Goksel A, Midilli K, Arisoy N, et al. Purified protein derivative response in juvenile idiopathic arthritis. J Rheumatol. 2009;36(9):2029–32.
Lu Y, Jacobson DL, Ashworth LA, Grand RJ, Meyer AL, McNeal MM, et al. Immune response to influenza vaccine in children with inflammatory bowel disease. Am J Gastroenterol. 2009;104(2):444–53.
Ogimi C, Tanaka R, Saitoh A, Oh-Ishi T. Immunogenicity of influenza vaccine in children with pediatric rheumatic diseases receiving immunosuppressive agents. Pediatr Infect Dis J. 2011;30(3):208–11.
Aytac MB, Kasapcopur O, Aslan M, Erener-Ercan T, Cullu-Cokugras F, Arisoy N. Hepatitis B vaccination in juvenile systemic lupus erythematosus. Clin Exp Rheumatol. 2011;29(5):882–6.
Aikawa NE, Campos LM, Silva CA, Carvalho JF, Saad CG, Trudes G, et al. Glucocorticoid: major factor for reduced immunogenicity of 2009 influenza A (H1N1) vaccine in patients with juvenile autoimmune rheumatic disease. J Rheumatol. 2012;39(1):167–73.
Campos LM, Silva CA, Aikawa NE, Jesus AA, Moraes JC, Miraglia J, et al. High disease activity: an independent factor for reduced immunogenicity of the pandemic influenza a vaccine in patients with juvenile systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2013;65(7):1121–7.
Heijstek MW, Pileggi GC, Zonneveld-Huijssoon E, Armbrust W, Hoppenreijs EP, Uiterwaal CS, et al. Safety of measles, mumps and rubella vaccination in juvenile idiopathic arthritis. Ann Rheum Dis. 2007;66(10):1384–7.
Borte S, Liebert UG, Borte M, Sack U. Efficacy of measles, mumps and rubella revaccination in children with juvenile idiopathic arthritis treated with methotrexate and etanercept. Rheumatology (Oxford). 2009;48(2):144–8.
Woerner A, Sauvain MJ, Aebi C, Otth M, Bolt IB. Immune response to influenza vaccination in children treated with methotrexate or/and tumor necrosis factor-alpha inhibitors. Hum Vaccin. 2011;7(12):1293–8.
Aikawa NE, Campos LM, Goldenstein-Schainberg C, Saad CG, Ribeiro AC, Bueno C, et al. Effective seroconversion and safety following the pandemic influenza vaccination (anti-H1N1) in patients with juvenile idiopathic arthritis. Scand J Rheumatol. 2013;42(1):34–40.
Tacke C, Smits G, van der Klis F et al. Reduced serologic response to mumps, measles, and rubella vaccination in patients treated with intravenous immunoglobulin for Kawasaki disease. J Allergy Clin Immunol. 2013;131(6):1701–3.
Lu Y, Bousvaros A. Varicella vaccination in children with inflammatory bowel disease receiving immunosuppressive therapy. J Pediatr Gastroenterol Nutr. 2010;50(5):562–5.
Farmaki E, Kanakoudi-Tsakalidou F, Spoulou V, Trachana M, Pratsidou-Gertsi P, Tritsoni M, et al. The effect of anti-TNF treatment on the immunogenicity and safety of the 7-valent conjugate pneumococcal vaccine in children with juvenile idiopathic arthritis. Vaccine. 2010;28(31):5109–13.
Erguven M, Kaya B, Hamzah OY, Tufan F. Evaluation of immune response to hepatitis A vaccination and vaccine safety in juvenile idiopathic arthritis. J Chin Med Assoc. 2011;74(5):205–8.
Dell’Era L, Corona F, Daleno C, Scala A, Principi N, Esposito S. Immunogenicity, safety and tolerability of MF59-adjuvanted seasonal influenza vaccine in children with juvenile idiopathic arthritis. Vaccine. 2012;30(5):936–40.
Shinoki T, Hara R, Kaneko U, Miyamae T, Imagawa T, Mori M, et al. Safety and response to influenza vaccine in patients with systemic-onset juvenile idiopathic arthritis receiving tocilizumab. Mod Rheumatol. 2012;22(6):871–6.
Malleson PN, Tekano JL, Scheifele DW, Weber JM. Influenza immunization in children with chronic arthritis: a prospective study. J Rheumatol. 1993;20(10):1769–73.
Hsu YH, Wang YH, Hsu WY, Lee YP. Kawasaki disease characterized by erythema and induration at the Bacillus Calmette–Guerin and purified protein derivative inoculation sites. Pediatr Infect Dis J. 1987;6(6):576–8.
Kuniyuki S, Asada M. An ulcerated lesion at the BCG vaccination site during the course of Kawasaki disease. J Am Acad Dermatol. 1997;37(2 Pt 2):303–4.
Antony D, Jessy PL. Involvement of BCG scar in Kawasaki disease. Indian Pediatr. 2005;42(1):83–4.
Weinstein M. Inflammation at a previous inoculation site: an unusual presentation of Kawasaki disease. CMAJ. 2006;174(4):459–60.
Chalmers D, Corban JG, Moore PP. BCG site inflammation: a useful diagnostic sign in incomplete Kawasaki disease. J Paediatr Child Health. 2008;44(9):525–6.
Uehara R, Igarashi H, Yashiro M, Nakamura Y, Yanagawa H. Kawasaki disease patients with redness or crust formation at the Bacille Calmette–Guerin inoculation site. Pediatr Infect Dis J. 2010;29(5):430–3.
Drachtman RA,Murphy S, Ettinger LJ. Exacerbation of chronic idiopathic thrombocytopenic purpura following measles-mumps-rubella immunization. Arch Pediatr Adolesc Med. 1994;148(3):326–7.
Korematsu S, Miyahara H, Kawano T, Yamada H, Akiyoshi K, Sato K, et al. A relapse of systemic type juvenile idiopathic arthritis after a rubella vaccination in a patient during a long-term remission period. Vaccine. 2009;27(37):5041–2.
Soybilgic A, Onel KB, Utset T, Alexander K, Wagner-Weiner L. Safety and immunogenicity of the quadrivalent HPV vaccine in female systemic lupus erythematosus patients aged 12 to 26 years. Pediatr Rheumatol Online J. 2013;11:29.
Beran J, Dedek P, Stepanova V, Spliio M, Pozler O. Safety and immunogenicity of a combined vaccine against hepatitis A and B in patients with autoimmune hepatitis. Cent Eur J Public Health. 2005;13(1):20–3.
Moses J, Alkhouri N, Shannon A, Feldstein A, Carter-Kent C. Response to hepatitis A vaccine in children with inflammatory bowel disease receiving infliximab. Inflamm Bowel Dis. 2011;17(12):E160.
Denman EJ, Denman AM, Greenwood BM, Gall D, Heath RB. Failure of cytotoxic drugs to suppress immune responses of patients with rheumatoid arthritis. Ann Rheum Dis. 1970;29(3):220–31.
Mamula P, Markowitz JE, Piccoli DA, Klimov A, Cohen L, Baldassano RN. Immune response to influenza vaccine in pediatric patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5(7):851–6.
Shimizu M, Ueno K, Yachie A. Relapse of systemic juvenile idiopathic arthritis after influenza vaccination in a patient receiving tocilizumab. Clin Vaccine Immunol. 2012;19(10):1700–2.
Guissa VR, Pereira RM, Sallum AM, Aikawa NE, Campos LM, Silva CA, et al. Influenza A H1N1/2009 vaccine in juvenile dermatomyositis: reduced immunogenicity in patients under immunosuppressive therapy. Clin Exp Rheumatol. 2012;30(4):583–8.
Zonneveld-Huijssoon E, Ronaghy A, Van Rossum MA, Rijkers GT, van der Klis FR, Sanders EA, et al. Safety and efficacy of meningococcal C vaccination in juvenile idiopathic arthritis. Arthritis Rheum. 2007;56(2):639–46.
Hoyeraal HM, Mellbye OJ. Humoral immunity in juvenile rheumatoid arthritis. Ann Rheum Dis. 1974;33(3):248–54.
Kashef S, Ghazizadeh F, Derakhshan A, Farjadian S, Alyasin S. Antigen-specific antibody response in juvenile-onset SLE patients following routine immunization with tetanus toxoid. Iran J Immunol. 2008;5(3):181–4.
Klumb EM, Araujo Jr ML, Jesus GR, Santos DB, Oliveira AV, Albuquerque EM, et al. Is higher prevalence of cervical intraepithelial neoplasia in women with lupus due to immunosuppression? J Clin Rheumatol. 2010;16(4):153–7.
Nath R, Mant C, Luxton J, Hughes G, Raju KS, Shepherd P, et al. High risk of human papillomavirus type 16 infections and of development of cervical squamous intraepithelial lesions in systemic lupus erythematosus patients. Arthritis Rheum. 2007;57(4):619–25.
Tam LS, Chan AY, Chan PK, Chang AR, Li EK. Increased prevalence of squamous intraepithelial lesions in systemic lupus erythematosus: association with human papillomavirus infection. Arthritis Rheum. 2004;50(11):3619–25.
Mok CC, Ho LY, Fong LS, To CH. Immunogenicity and safety of a quadrivalent human papillomavirus vaccine in patients with systemic lupus erythematosus: a case-control study. Ann Rheum Dis. 2013;72(5):659–64.
Che H, Lukas C, Morel J, Combe B. Risk of herpes/herpes zoster during anti-tumor necrosis factor therapy in patients with rheumatoid arthritis. Systematic review and meta-analysis. Joint Bone Spine. 2014;81(3):215–21.
Zhang J, Xie F, Delzell E, Chen L, Winthrop KL, Lewis JD, et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA. 2012;308(1):43–9. Illustrates the importance of vaccination against HZ in a large cohort of adult RD patients.
Arnheim-Dahlstrom L, Pasternak B, Svanstrom H, Sparen P, Hviid A. Autoimmune, neurological, and venous thromboembolic adverse events after immunisation of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: cohort study. BMJ. 2013;347:f5906. Illustrates the safety of the HPV vaccine in a large cohort of healthy children, which was debated in previous, smaller studies.
Marciano BE, Huang CY, Joshi G, Rezaei N, Carvalho BC, Allwood Z, et al. BCG vaccination in patients with severe combined immunodeficiency: complications, risks, and vaccination policies. J Allergy Clin Immunol. 2014;133(4):1134–41.
Cheent K, Nolan J, Shariq S, Kiho L, Pal A, Arnold J. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4(5):603–5.
Scheinberg M, Guedes-Barbosa LS, Mangueira C, Rosseto EA, Mota L, Oliveira AC, et al. Yellow fever revaccination during infliximab therapy. Arthritis Care Res (Hoboken). 2010;62(6):896–8.
Bach JF. Protective role of infections and vaccinations on autoimmune diseases. J Autoimmun. 2001;16(3):347–53.
Mills KH. TLR-dependent T cell activation in autoimmunity. Nat Rev Immunol. 2011;11(12):807–22.
von Herrath MG, Fujinami RS, Whitton JL. Microorganisms and autoimmunity: making the barren field fertile? Nat Rev Microbiol. 2003;1(2):151–7.
Wraith DC, Goldman M, Lambert PH. Vaccination and autoimmune disease: what is the evidence? Lancet. 2003;362(9396):1659–66.
Shoenfeld Y, Agmon-Levin N. ‘ASIA’—autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011;36(1):4–8.
Pellegrino P, Perrone V, Pozzi M, Carnovale C, Perrotta C, Clementi E, et al. The epidemiological profile of ASIA syndrome after HPV vaccination: an evaluation based on the Vaccine Adverse Event Reporting Systems. Immunol Res. 2015;61(1–2):90–6.
Verstraeten T, Descamps D, David MP, Zahaf T, Hardt K, Izurieta P, et al. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine. 2008;26(51):6630–8.
Isai A, Durand J, Le Meur S, Hidalgo-Simon A, Kurz X. Autoimmune disorders after immunisation with Influenza A/H1N1 vaccines with and without adjuvant: EudraVigilance data and literature review. Vaccine. 2012;30(49):7123–9.
Zielinski CE, Corti D, Mele F, et al. Dissecting the human immunologic memory for pathogens. Immunol Rev. 2011;240:40–51.
Carollo M, Palazzo R, Bianco M, et al. Hepatitis B specific T cell immunity induced by primary vaccination persists independently of the protective serum antibody level. Vaccine. 2013;31:506–13.
Compliance with Ethics Guidelines
Conflict of Interest
N. Groot and Dr. M.W. Heijstek have no conflicts of interest to declare. Prof. Dr. Wulffraat reports unrestricted educational grants from the Dutch Arthritis Foundation and GlaxoSmithKline.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Pediatric Rheumatology
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Groot, N., Heijstek, M.W. & Wulffraat, N.M. Vaccinations in Paediatric Rheumatology: an Update on Current Developments. Curr Rheumatol Rep 17, 46 (2015). https://doi.org/10.1007/s11926-015-0519-y
Published:
DOI: https://doi.org/10.1007/s11926-015-0519-y