Abstract
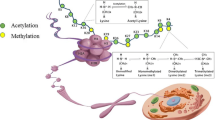
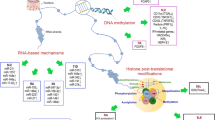
The multiple inter-dependent post-translational modifications of histones represent fine regulators of chromatin dynamics. These covalent modifications, including phosphorylation, acetylation, ubiquitination, deimination, and methylation, affect therefore the numerous processes involving chromatin, such as replication, repair, transcription, genome stability, and cell death. Specific enzymes introducing modified residues in histones are precisely regulated, and a single amino acid residue can be subjected to a single or several, independent modifications. Disruption of histone post-translational modifications perturbs the pattern of gene expression, which may result in disease manifestations. It has become evident in recent years that apoptosis-modified histones exert a central role in the induction of autoimmunity, for example in systemic lupus erythematosus and rheumatoid arthritis. Certain histone post-translational modifications are linked to cell death (apoptotic and non-apoptotic cell death) and might be involved in lupus in the activation of normally tolerant lymphocyte subpopulations. In this review, we discuss how these modifications can affect the antigenicity and immunogenicity of histones with potential consequences in the pathogenesis of autoimmune diseases.


Similar content being viewed by others
References
Bhaumik SR, Smith E, Shilatifard A (2007) Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol 14:1008–1016
Ruthenburg AJ, Li H, Patel DJ, Allis CD (2007) Multivalent engagement of chromatin modifications by linked binding modules. Nat Rev Mol Cell Biol 8:983–994
Mahler M, Blüthner M, Pollard KM (2003) Advances in B-cell epitope analysis of autoantigens in connective tissue diseases. Clin Immunol 107:65–79
Routsias JG, Tzioufas AG, Moutsopoulos HM (2004) The clinical value of intracellular autoantigens B-cell epitopes in systemic rheumatic diseases. Clin Chim Acta 340:1–25
LeFeber WP, Norris DA, Ryan SR et al (1984) Ultraviolet light induces binding of antibodies to selected nuclear antigens on cultured human keratinocytes. J Clin Invest 74:1545–1551
Casciola-Rosen LA, Anhalt G, Rosen A (1994) Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med 179:1317–1330
Casiano CA, Tan EM (1996) Recent developments in the understanding of antinuclear autoantibodies. Int Arch Allergy Immunol 111:308–313
Utz PJ, Gensler TJ, Anderson P (2000) Death, autoantigen modifications, and tolerance. Arthritis Res 2:101–114
Rodenburg RJT, Raats HMJ, Pruijn GJM, van Venrooij WJ (2000) Cell death: a trigger of autoimmunity? BioEssay 22:627–636
Rovere P, Sabbadini MG, Fazzini F et al (2000) Remnants of suicidal cells fostering systemic autoaggression. Apoptosis in the origin and maintenance of autoimmunity. Arthritis Rheum 43:1663–1672
Gaipl US, Kuhn A, Sheriff A et al (2006) Clearance of apoptotic cells in human SLE. Curr Dir Autoimmun 9:173–187
Munoz LE, van Bavel C, Franz S, Berden J, Herrmann M, van der Vlag J (2008) Apoptosis in the pathogenesis of systemic lupus erythematosus. Lupus 17:371–375
Dumortier H, Muller S (2007) Histone autoantibodies. In: Shoenfeld Y, Meroni PL, Gershwin E (eds) Textbook of autoantibodies, chapter 22, 2nd edn. Elsevier, Amsterdam, pp 169–176
Cheung WL, Ajiro K, Samejima K et al (2003) Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase. Cell 113:507–517
Monestier M, Fasy TM, Losman MJ, Novick KE, Muller S (1993) Structure and binding properties of monoclonal antibodies to core histones from autoimmune mice. Mol Immunol 30:1069–1075
Monestier M, Decker P, Briand JP, Gabriel JL, Muller S (2000) Molecular and structural properties of three autoimmune IgG monoclonal antibodies to histone H2B. J Biol Chem 275:13558–13563
Fournel S, Muller S (2003) Synthetic peptides in the diagnosis of systemic autoimmune diseases. Curr Prot Peptide Sci 4:261–276
Lu L, Kaliyaperumal A, Boumpas DT, Datta SK (1999) Major peptide autoepitopes for nucleosome-specific T cells of human lupus. J Clin Invest 104:345–355
Kaliyaperumal A, Mohan C, Wu W, Datta SK (1996) Nucleosomal peptide epitopes for nephritis-inducing T helper cells of murine lupus. J Exp Med 183:2459–2469
Sköldberg F, Rönnblom L, Thornemo M et al (2002) Identification of AHNAK as a novel autoantigen in systemic lupus erythematosus. Biochem Biophys Res Commun 291:951–958
Stemmer C, Briand JP, Muller S (1994) Mapping of linear epitopes of human histone H1 recognized by rabbit anti-H1/H5 antisera and antibodies from autoimmune patients. Mol Immunol 31:1037–1046
Dieker JW, Fransen JH, Van Bavel CC et al (2007) Apoptosis-induced acetylation of histones is pathogenic in systemic lupus erythematosus. Arthritis Rheum 56:1921–1933
Batova I, Kowal C, May R, Scharff MD, Diamond B (2008) Human recombinant Fab fragments with sub-nanomolar affinities for acetylated histones. J Immunol Methods 329:1–10
Hu N, Qiu X, Luo Y et al (2008) Abnormal histone modifications patterns in lupus CD4+ T cells. J Rheumatol 35:804–810
Garcia BA, Busby SA, Shabanowitz J, Hunt DF, Mishra N (2005) Resetting the epigenetic histone code in the MRL-lpr/lpr mouse model of lupus by histone acetylase inhibition. J Proteome Res 4:2032–2042
Mishra N, Reilly CM, Brown DR, Ruiz P, Gilkeson GS (2003) Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J Clin Invest 111:539–552
Reilly CM, Mishra N, Miller JM et al (2004) Modulation of renal disease in MRL/lpr mice by suberoylanilide hydroxamic acid. J Immunol 173:4171–4178
Marushige Y, Marushige K (1995) Disappearance of ubiquitinated histone H2A during chromatin condensation in TGF beta 1-induced apoptosis. Anticancer Res 15:267–272
Mimnaugh EG, Kayastha G, McGovern NB et al (2001) Caspase-dependent deubiquitination of monoubiquitinated nucleosomal histone H2A induced by diverse apoptogenic stimuli. Cell Death Differ 8:1182–1196
Plaué S, Muller S, Van Regenmortel MHV (1989) A branched, synthetic octapeptide of ubiquinated histone H2A as target of autoantibodies. J Exp Med 169:1607–1617
Muller S, Briand JP, Van Regenmortel MHV (1988) Presence of antibodies to ubiquitin during the autoimmune response associated with systemic lupus erythematosus. Proc Natl Acad Sci USA 85:8176–8180
Ravirajan CT, Kalsi J, Winska-Wiloch H et al (1992) Antigen binding diversity of human hybridoma autoantibodies derived from splenocytes of patients with SLE. Lupus 1:157–165
Stöckl F, Muller S, Batsford S et al (1994) A role for histones and ubiquitin in lupus nephritis? Clin Nephrol 41:10–17
Elouaai F, Lule J, Benoist H et al (1994) Autoimmunity to histones, ubiquitin, and ubiquitinated histone H2A in NZB x NZW an MRL-lpr/lpr mice. Anti-histone antibodies are concentrated in glomerular eluates of lupus mice. Nephrol Dialysis Transpl 9:362–366
Mézière C, Stöckl F, Batsford S, Vogt A, Muller S (1994) Antibodies to DNA, chromatin core particles and histones in mice with graft-versus-host disease and their involvement in glomerular injury. Clin Exp Immunol 98:287–294
Reumaux D, Mézière C, Colombel JF, Duthilleul P, Muller S (1995) Distinct production of antibodies to nuclear components in ulcerative colitis and Crohn’s disease. Clin Immunol Immunopathol 77:349–357
Kanai Y, Kawaminami Y, Miwa M et al (1977) naturally-occurring antibodies to poly(ADP-ribose) in patients with systemic lupus erythematosus. Nature 265:175–177
Muller S, Briand JP, Barakat S et al (1994) Autoantibodies reacting with poly(ADP-ribose) and with a zinc-finger functional domain of poly(ADP-ribose)polymerase involved in the recognition of damaged DNA. Clin Immunol Immunopathol 73:187–196
Decker P, Briand JP, De Murcia G, Pero RW, Isenberg DA, Muller S (1998) Zinc is an essential co-factor for recognition of the DNA-binding domain of poly(ADP-ribose) polymerase by antibodies in autoimmune rheumatic and bowel diseases. Arthritis Rheum 41:918–926
Thibeault L, Hengartner M, Lagueux J, Poirier G, Muller S (1992) Rearrangement of the nucleosome structure in chromatin by poly (ADP-ribose). Biochim Biophys Acta 1121:317–324
Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ (2003) PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays 25:1106–1118
Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ (1998) Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest 101:273–281
Girbal-Neuhauser E, Durieux JJ, Arnaud M et al (1999) The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J Immunol 162:585–594
Masson-Bessière C, Sebbag M, Girbal-Neuhauser E et al (2001) The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J Immunol 166:4177–4184
Cuthbert GL, Daujat S, Snowden AW et al (2004) Histone deimination antagonizes arginine methylation. Cell 118:545–553
Wang Y, Wysocka J, Sayegh J et al (2004) Human PAD4 regulates histone arginine methylation levels via demethylimination. Science 306:279–283
Wang Y, Li M, Stadler S et al (2009) Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 184:205–213
Neeli I, Khan SN, Radic M (2008) Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol 180:1895–1902
Brahms H, Raymackers J, Union A, de Keyser F, Meheus L, Lührmann R (2000) The C-terminal RG dipeptide repeats of the spliceosomal Sm proteins D1 and D3 contain symmetrical dimethylarginines, which form a major B-cell epitope for anti-Sm autoantibodies. J Biol Chem 275:17122–17129
Mahler M, Stinton LM, Fritzler MJ (2005) Improved serological differentiation between systemic lupus erythematosus and mixed connective tissue disease by use of an SmD3 peptide-based immunoassay. Clin Diagn Lab Immunol 12:107–113
Mahler M, Fritzler MJ, Blüthner M (2005) Identification of a SmD3 epitope with a single symmetrical dimethylation of an arginine residue as a specific target of a subpopulation of anti-Sm antibodies. Arthritis Res Ther 7:R19–R29
Piacentini M, Colizzi V (1999) Tissue transglutaminase: apoptosis versus autoimmunity. Immunol Today 20:130–134
Khan MA, Dixit K, Jabeen S, Moinuddin AK (2009) Impact of peroxynitrite modification on structure and immunogenicity of H2A histone. Scand J Immunol 69:99–109
Young GW, Hoofring SA, Mamula MJ et al (2005) Structural integrity of histone H2B in vivo requires the activity of protein L-isoaspartate O-methyltransferase, a putative protein repair enzyme. J Biol Chem 280:26094–26098
Muller S, Isabey A, Couppez M, Plaué S, Sommermeyer G, Van Regenmortel MHV (1987) Specificity of antibodies raised against triacetylated histone H4. Mol Immunol 24:779–789
Kuo WN, Kreahling JM, Shanbhag VP, Shanbhag PP, Mewar M (2000) Protein nitration. Mol Cell Biochem 214:121–129
Kono H, Rock KL (2008) How dying cells alert the immune system to danger. Nat Rev Immunol 8:279–289
Urbonaviciute V, Fürnrohr BG, Meister S et al (2008) Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med 205:3007–3018
Dieker JW, van der Vlag J, Berden JH (2002) Triggers for anti-chromatin autoantibody production in SLE. Lupus 11:856–864
Arnheim N, Calabrese P (2009) Understanding what determines the frequency and pattern of human germline mutations. Nat Rev Genet 10:478–488
Barros SP, Offenbacher S (2009) Epigenetics: connecting environment and genotype to phenotype and disease. J Dent Res 88:400–408
Figueiredo LM, Cross GA, Janzen CJ (2009) Epigenetic regulation in African trypanosomes: a new kid on the block. Nat Rev Microbiol 7:504–513
Hewagama A, Richardson B (2009) The genetics and epigenetics of autoimmune diseases. J Autoimmun 33:3–11
Invernizzi P (2009) Future directions in genetic for autoimmune diseases. J Autoimmun 33:1–2
Invernizzi P, Pasini S, Selmi C, Gershwin ME, Podda M (2009) Female predominance and X chromosome defects in autoimmune diseases. J Autoimmun 33:12–16
Larizza D, Calcaterra V, Martinetti M (2009) Autoimmune stigmata in Turner syndrome: when lacks an X chromosome. J Autoimmun 33:25–30
Persani L, Rossetti R, Cacciatore C, Bonomi M (2009) Primary Ovarian Insufficiency: X chromosome defects and autoimmunity. J Autoimmun 33:35–41
Sawalha AH, Harley JB, Scofield RH (2009) Autoimmunity and Klinefelter's syndrome: when men have two X chromosomes. J Autoimmun 33:31–34
Wells AD (2009) New insights into the molecular basis of T cell anergy: anergy factors, avoidance sensors, and epigenetic imprinting. J Immunol 182:7331–7341
Zernicka-Goetz M, Morris SA, Bruce AW (2009) Making a firm decision: multifaceted regulation of cell fate in the early mouse embryo. Nat Rev Genet 10:467–477
Acknowledgments
This work was supported by the French Centre National de la Recherche Scientifique (CNRS). JD was supported by a post-doctoral fellowship from CNRS and the French Fondation pour la Recherche Médicale.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dieker, J., Muller, S. Epigenetic Histone Code and Autoimmunity. Clinic Rev Allerg Immunol 39, 78–84 (2010). https://doi.org/10.1007/s12016-009-8173-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-009-8173-7