Abstract
Background
Persistence is commonly considered a key factor for the successful management of osteoporosis and fragility fractures. Denosumab is the first biologic agent developed for the treatment of osteoporosis with satisfying data regarding the persistence with this therapy.
Aim
The purpose of this multicenter observational real practice study was to evaluate the persistence with denosumab treatment in post-menopausal women affected by osteoporosis.
Material/subjects and methods
Women were recruited in four specialized centers for the management of osteoporosis in North, Center and South of Italy. We included women with a diagnosis of post-menopausal osteoporosis, aged >50 years, able to obtain a prescription according to the Italian reimbursement criteria in force during the study period for anti-osteoporotic pharmacological treatment. They initiated a treatment with subcutaneous denosumab (Prolia®) 60 mg/every 6 months between November 2011 and May 2016. Women who had received aromatase inhibitors were excluded. Patients were assessed at baseline and every 6 months for all treatment length. Persistence data were evaluated for a total of 36 months.
Results
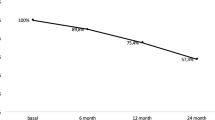
Eight hundred seventy women were enrolled; mean aged 70 years, with a mean body mass index of 24.8 ± 4.1 kg/m2. At the Dual-energy X-ray absorptiometry assessment, the mean lumbar spine T-score was −2.76 ± 1.14 standard deviations (SD) and the mean femoral neck T-score was −2.49 ± 0.80 SD. During the study, the total persistence was 91.4%. Total dropouts were 75 (8.6%), higher within the initial 6-month period of treatment.
Conclusions
Persistence to denosumab treatment in our observational real practice study was very high. These results suggest that factors such as frequency of visits, pharmacological schedule, and opportunity to call the doctor might play an important role in the persistence and adherence to treatment to obtain maximum therapeutic effect and avoid further fragility fractures.

Similar content being viewed by others
Abbreviations
- BPs:
-
Bisphosphonates
- PMO:
-
Post-menopausal osteoporosis
- RCTs:
-
Randomized clinical trials
- BMD:
-
Bone mineral density
- HRQoL:
-
Health related quality of life
- FN:
-
Femoral neck
- SD:
-
Standard deviations
- LS:
-
Lumbar spine
References
Cooper C (1997) The crippling consequences of fractures and their impact on quality of life. Am J Med 103(Suppl):12–17
Oleksik A, Lips P, Dawson A et al (2000) Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J Bone Miner Res 15(7):1384–1392
McClung M, Harris ST, Miller PD et al (2013) Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med 126(1):13–20
Vescini F, Attanasio R, Balestrieri A et al (2016) Italian association of clinical endocrinologists (AME) position statement: drug therapy of osteoporosis. J Endocrinol Invest 39(7):807–834
Cairoli E, Zhukouskaya VV, Eller-Vainicher C, Chiodini I (2015) Perspectives on osteoporosis therapies. J Endocrinol Invest 38(3):303–311
Silverman SL, Gold DT, Cramer JA (2007) Reduced fracture rates observed only in patients with proper persistence and compliance with bisphosphonate therapies. South Med 100(1214–1218):10
Adler RA (2016) Osteoporosis treatment: complexities and challenges. J Endocrinol Invest 39:719–720
Migliaccio S, Resmini G, Buffa A et al (2013) Evaluation of persistence and adherence to teriparatide treatment in patients affected by severe osteoporosis (PATT): a multicenter observational real life study. Clin Cases Miner Bone Metab 10(1):56–60
Cummings SR, San Martin J, McClung MR, FREEDOM Trial et al (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361(8):756–765
Hadji P, Papaioannou N, Gielen E et al (2015) Persistence, adherence, and medication-taking behavior in women with postmenopausal osteoporosis receiving denosumab in routine practice in Germany, Austria, Greece, and Belgium: 12-month results from a European non-interventional study. Osteoporos Int 26(10):2479–2489
Lagari VS, McAninch E, Baim S (2015) Considerations regarding adherence of anti-osteoporosis therapy. Postgrad Med 127(1):92–98
Silverman SL, Siris E, Kendler DL et al (2015) Persistence at 12 months with denosumab in postmenopausal women with osteoporosis: interim results from a prospective observational study. Osteoporos Int 26(1):361–372
Kendler DL, Macarios D, Lillestol MJ et al (2014) Influence of patient perceptions and preferences for osteoporosis medication on adherence behavior in the denosumab adherence preference satisfaction study. Menopause 21(1):25–32
Ringe JD, Farahmand P (2014) Improved real-life adherence of 6-monthly denosumab injections due to positive feedback based on rapid 6-month BMD increase and good safety profile. Rheumatol Int 34(5):727–732
Reginster JY (2006) Adherence and persistence: impact on outcomes and health care resources. Bone 38(Suppl2):18–21
Carr AJ, Thompson PW, Cooper C (2006) Factors associated with adherence and persistence to bisphosphonate therapy in osteoporosis: a cross-sectional survey. Osteoporos Int 17:1638–1644
Recker RR, Gallagher R, MacCosbe PE (2005) Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin Proc 80:856–861
Penning-van Beest FJ, Goettsch WG, Erkens JA, Herings RM (2006) Determinants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosis. Clin Ther 28:236–242
Clowes JA, Peel NF, Eastell R (2004) The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab 89:1117–1123
Fuksa L, Vytrisalova M (2015) Adherence to denosumab in the treatment of osteoporosis and its utilization in the Czech Republic. Curr Med Res Opin 31(9):1645–1653
AIFA (2006–2007) Notes for the appropriate use of medicinal products. Official Gazette 2007; Suppl 7:61
Cramer JA, Roy A, Burrell A et al (2008) Medication compliance and persistence: terminology and definitions. Value Health 11(1):44–47
Yeaw J, Benner JS, Walt JG et al (2009) Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm 15(9):728–740
Ross S, Samuels E, Gairy K et al (2011) A meta-analysis of osteoporotic fracture risk with medication nonadherence. Value Health 14(4):571–581
Lewiecki M, Binkley N (2016) What we don’t know about osteoporosis. J Endocrinol Invest 39(5):491–493
Yood RA, Emani S, Reed JI et al (2003) Compliance with pharmacologic therapy for osteoporosis. Osteoporos Int 14(12):965–968
Siris ES, Selby PL, Saag KG et al (2009) Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med 122(2 Suppl):S3–S13
Kothawala P, Badamgarav E, Ryu S et al (2007) Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc 82(12):1493–1501
Iolascon G, Gimigliano F, Moretti A et al (2016) Rates and reasons for lack of persistence with anti-osteoporotic drugs: analysis of the Campania region database. Clin Cases Miner Bone Metab 13(2):127–130
Blouin J, Dragomir A, Fredette M et al (2009) Comparison of direct health care costs related to the pharmacological treatment of osteoporosis and to the management of osteoporotic fractures among compliant and noncompliant users of alendronate and risedronate: a population-based study. Osteoporos Int 20(9):1571–1581
Siris ES, Pasquale MK, Wang Y, Watts NB (2011) Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001–2008. J Bone Miner Res 26(1):3–11
Rossini M, Bianchi G, Di Munno O et al (2006) Treatment of Osteoporosis in clinical Practice (TOP) Study Group. Determinants of adherence to osteoporosis treatment in clinical practice. Osteoporos Int 17(6):914–921
Karlsson L, Lundkvist J, Psachoulia E et al (2015) Persistence with denosumab and persistence with oral bisphosphonates for the treatment of postmenopausal osteoporosis: a retrospective, observational study, and a meta-analysis. Osteoporos Int 26(10):2401–2411
Kendler DL, Roux C, Benhamou CL et al (2010) Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 25(1):72–81
Brown JP, Prince RL, Deal C et al (2009) Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res 24(1):153–161
Silverman SL, Siris E, Kendler DL et al (2015) Persistence at 12 months with denosumab in postmenopausal women with osteoporosis: interim results from a prospective observational study. Osteoporos Int 26(1):361–372
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
The study received the approval of the Internal Review Board of each Institution involved.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Migliaccio, S., Francomano, D., Romagnoli, E. et al. Persistence with denosumab therapy in women affected by osteoporosis with fragility fractures: a multicenter observational real practice study in Italy. J Endocrinol Invest 40, 1321–1326 (2017). https://doi.org/10.1007/s40618-017-0701-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-017-0701-3