Key Points
-
Interleukin-2 (IL-2) was discovered as a cytokine that supports the proliferation and differentiation of effector T cells. IL-2 initially entered clinical development based on this activity, in settings such as cancer and infectious diseases.
-
When used at high doses in patients with melanoma or renal cell carcinoma, IL-2 induces relatively rare (around 7%) but durable complete responses, at the expense of severe side effects. IL-2 has been approved by the US Food and Drug Administration for these indications.
-
Surprisingly, knockout of the genes encoding IL-2 or IL-2 receptor in mice led to severe autoimmunity, rather than the predicted immune deficiency. This was later explained by a defect in regulatory T (TReg) cells, and the discovery that IL-2 is the key cytokine for TReg cell function and survival.
-
Further studies showed that IL-2 also favours the development of activated CD4+ T cells towards the T helper 1 (TH1), TH2, TH9 and peripherally induced TReg (pTReg) cell lineages, rather than the TH17 and T follicular helper (TFH) cell lineages. Thus, IL-2 contributes to tipping the immune balance towards regulation rather than inflammation, notably by favouring the differentiation of pTReg cells over TH17 cells; helps to control autoantibody generation by favouring T follicular regulatory cells over TFH cells; and helps to control autoreactive CD8+ effector T cells.
-
Proof-of-concept clinical trials have shown that at a low dose, IL-2 is well tolerated, induces TReg cells and mediates clinical improvements in autoimmune and inflammatory diseases; these findings have been confirmed in additional trials.
-
These trials and additional experimental work also showed that IL-2 mediates immunoregulation without immunosuppression. This opens the door for broad investigation of the therapeutic potential of low-dose IL-2 in a large number of autoimmune and inflammatory diseases.
Abstract
Depletion of regulatory T (TReg) cells in otherwise healthy individuals leads to multi-organ autoimmune disease and inflammation. This indicates that in a normal immune system, there are self-specific effector T cells that are ready to attack normal tissue if they are not restrained by TReg cells. The data imply that there is a balance between effector T cells and TReg cells in health and suggest a therapeutic potential of TReg cells in diseases in which this balance is altered. Proof-of-concept clinical trials, now supported by robust mechanistic studies, have shown that low-dose interleukin-2 specifically expands and activates TReg cell populations and thus can control autoimmune diseases and inflammation.
Similar content being viewed by others
Main
To avoid immune responses against self-antigens, most self-reactive T cells are eliminated during selection in the thymus. However, this selection process is 'leaky', and potentially harmful self-specific T cells with possible effector activity are released from the thymus. Self-specific T cells do not usually mediate autoimmune diseases because, in a normal immune system, they are suppressed in the periphery by thymus-derived regulatory T (TReg) cells1,2. This TReg cell-mediated control is exemplified by three observations: first, humans and mice with a genetically determined TReg cell deficit develop multiple organ-specific autoimmune diseases3,4; second, a quantitative or qualitative defect in TReg cells is present in many mouse and human autoimmune diseases5; and third, efficient depletion of TReg cells in healthy individuals at any time in life induces a rapid autoimmune and inflammatory response that leads to multi-organ autoimmunity6. These observations have led to the concept of a balance between TReg cells and effector T cells in health that is dysregulated in autoimmune diseases and inflammatory disorders, which have a TReg cell insufficiency.
The discovery of TReg cells has opened up a new therapeutic possibility in terms of restoring a healthy balance between TReg cells and effector T cells through a qualitative and/or quantitative increase in TReg cell function. Owing to a lack of drugs that were capable of enhancing TReg cell activity in vivo, initial efforts at therapeutic intervention used adoptively transferred TReg cells. Clinical improvements after TReg cell transfer have been seen in many animal models of autoimmune diseases and, recently, in humans with type 1 diabetes7.
The discovery that interleukin-2 (IL-2) is a key cytokine for TReg cell differentiation, survival and function8,9,10 has led to new opportunities for tipping the balance of TReg cells and effector T cells towards TReg cells. Proof-of-concept clinical trials have shown that at low doses, IL-2 specifically and safely activates TReg cells in humans, and also improves autoimmune and alloimmune inflammatory conditions11,12. These data inspired a broad investigation of the effects of IL-2 in diseases with an immune component, even beyond the field of autoimmunity.
Here, we review historical and current developments in IL-2 therapy, discussing evidence from mechanistic studies and clinical trials that has led to a shift in our understanding of IL-2 from a cytokine that activates effector T cells against cancer when used at a high dose, to a cytokine that activates TReg cells to control autoimmunity and inflammation at a low dose.
IL-2 and homeostasis
IL-2 was the first cytokine to be molecularly cloned, and based on its originally discovered action, it was named 'T cell growth factor' (Box 1). Many studies in the 1970s and 1980s convincingly showed that IL-2 is an autocrine survival and proliferation signal for T cells, and stimulates the differentiation of naive T cells into effector T cells13. Thus, IL-2 was established as the essential soluble mediator of T cell-dependent effector immune responses and was thought to be required for the development of all such responses.
The birth of an IL-2 paradox. The aforementioned dogma was not challenged until 1993, when it was discovered that knockout of the gene encoding IL-2 in mice led to severe lymphoproliferation and autoimmunity, rather than the predicted immune deficiency14. This finding was soon followed by the description of similar phenotypes of mice with a knockout of the gene encoding the β-chain of the IL-2 receptor (IL-2Rβ; hereafter referred to as CD122)15 or IL-2Rα (hereafter referred to as CD25)16. The description of CD25 as the canonical phenotypic marker of TReg cells17 suggested that IL-2 signalling defects might affect TReg cells and lead to autoimmunity. This was confirmed by showing that TReg cells are absent in IL-2-deficient or IL-2R-deficient mice, and that adoptive transfer of these cells from normal mice could prevent autoimmunity in the deficient mice18. The surprising conclusion of these knockout mouse studies is that the dominant role of IL-2 is the maintenance of TReg cells, and not the development of effector and memory T cells, because loss of IL-2 signalling leads to a breakdown of immune tolerance and homeostasis. Thus, an agent that had been used for decades to stimulate effector T cells turned out to be dispensable for these cells, and instead is required for activation of the TReg cells that suppress effector T cells.
Pleiotropic effects of IL-2. IL-2 is predominantly produced by activated CD4+ T cells and, to a lesser extent, by activated CD8+ T cells, activated dendritic cells, natural killer (NK) cells and NKT cells10. IL-2 production following activation of CD4+ T cells is transient; it involves increased transcription and stability of IL2 mRNA, followed by transcriptional silencing and degradation of IL2 mRNA8.
Three polypeptide chains — CD25, CD122 and IL-2Rγ (CD132) — together form the high-affinity IL-2R (dissociation constant (Kd) = 10−11 M)10. The IL-2-induced oligomerization of IL-2R initiates signal transduction, which is mediated only by the β-chain and γ-chain, through Janus kinase 1 (JAK1)-mediated and JAK3-mediated activation of signal transducer and activator of transcription 5 (STAT5), as well as the phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways. The CD122–CD132 dimer can also signal in the absence of CD25, but has a lower binding affinity for IL-2 (Kd = 10−9 M)10. The high-affinity trimeric IL-2R is constitutively expressed by TReg cells, whereas other types of T cell only acquire CD25 upon activation10.
IL-2 has pleiotropic functions (Fig. 1). It supports the development of TReg cells in the thymus, although it may be dispensable for this role19. It is a key survival factor for TReg cells in the periphery, and is required for the functional competence and stability of TReg cell populations20,21. The molecular mechanism of IL-2-induced TReg cell stability involves, at least in part, epigenetic changes in the FOXP3 locus (which encodes forkhead box P3)22,23. One canonical feature of FOXP3+ TReg cells is their inability to produce IL-2 (Refs 22,23).
Upon T cell receptor ligation and co-stimulation, naive CD4+ or CD8+ T cells transiently express CD25 and respond to interleukin-2 (IL-2) through the high-affinity trimeric IL-2 receptor (IL-2R). The differentiation fate of CD4+ T cells depends on the cytokine milieu: IL-12 and IL-18 favour the differentiation of T helper 1 (TH1) cells; IL-4 and IL-33 favour TH2 cells; transforming growth factor-β (TGFβ) and IL-4 favour TH9 cells; TGFβ, IL-1, IL-6 and IL-23 favour TH17 cells; TGFβ favours peripherally induced regulatory T (pTReg) cells; and IL-6 and IL-21 favour T follicular helper (TFH) cells. As part of the fate-orienting milieu, IL-2 favours the differentiation of TH1, TH2, TH9 and pTReg cells, and inhibits the differentiation of TH17 and TFH cells. The differentiation fate of CD4+ and CD8+ T cells is influenced by the strength of IL-2 signalling, with greater signal strength favouring differentiation into pTReg cells and effector memory T cells, respectively. TReg cells constitutively express CD25 and respond vigorously to IL-2 by upregulating CD25 and becoming more potent (activated) suppressor cells. Some of these cells migrate to lymph node germinal centres where they are known as T follicular regulatory (TFR) cells. Together, these changes contribute to tipping the immune balance towards regulation rather than inflammation, notably by: favouring the differentiation of naive CD4+ T cells into pTReg cells rather than TH17 cells (part a); helping to control autoantibody production by favouring the differentiation of TFR cells over TFH cells (part b); and helping to control autoreactive CD8+ effector T cells by increasing the number and function of TReg cells (part c). The mechanisms of the suppressive activity of CD8+ TReg cells in vivo remain to be determined.
Aside from its role in supporting TReg cells, the other main functions of IL-2 are to support the proliferation of CD4+ T cells, and to support the terminal differentiation of CD8+ T cells. In the absence of IL-2 signalling, CD8+ T cells respond to a primary infection but fail to respond to secondary antigen exposure24. Prolonged CD25 expression on CD8+ T cells favours terminal effector differentiation in vivo25,26. However, IL-2 neutralization induces autoimmune diseases such as gastritis, diabetes, thyroiditis and neuropathy, which could be adoptively transferred by CD4+ and CD8+ T cells27, suggesting that the differentiation of effector T cells can occur even in the absence of IL-2.
IL-2 has been shown to promote the development of naive CD4+ T cells into peripherally derived TReg cells28, T helper 2 (TH2) cells29, TH9 cells30 and, in some studies, TH1 cells31. By contrast, IL-2 suppresses the differentiation of CD4+ T cells into TH17 cells32 and T follicular helper (TFH) cells33 by a STAT5-dependent mechanism. Thus, IL-2 may suppress germinal centre formation and create a regulatory environment in germinal centres by altering the balance of T follicular regulatory cells (TFR cells)34,35,36 and TFH cells.
NK cells express high levels of the CD122–CD132 dimeric IL-2R8. IL-2 mainly stimulates the proliferation of NK cells, and also increases their cytotoxic activity and cytokine production, although to a lesser extent than does IL-12. Type 2 innate lymphoid cells (ILC2s) express high levels of CD25 (Ref. 37) and therefore proliferate in response to IL-2 through activation of the trimeric IL-2R38. This proliferation is accompanied by the production of IL-5, which can, in turn, expand eosinophil populations39. Following stimulation with IL-2, γδ T cells acquire interferon-γ production and cytolytic activity40.
In summary, although many studies show multiple effects of IL-2 on a range of immune cell populations, the most consistent finding of studies in which IL-2 is administered or inhibited is an effect on TReg cells, and this is the main focus of the remainder of this Review.
High sensitivity of TReg cells to IL-2. One of the most important results that has contributed to our understanding of the biology and therapeutic effects of IL-2 is the demonstration that IL-2-induced signalling and downstream gene activation occur at markedly lower concentrations of IL-2 in TReg cells than in effector T cells or NK cells41 (Fig. 2). TReg cells have a 10–20-fold lower activation threshold for IL-2 than effector T cells when measured in terms of the level of phosphorylated STAT5 (pSTAT5). Moreover, downstream of pSTAT5, the activation of numerous genes that are important for cell function requires IL-2 doses that are 100-times lower for TReg cells than for effector T cells42.
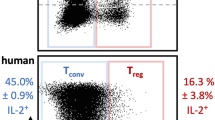
The activation of effector T cells and regulatory T (TReg) cells is mediated by combined signalling through the T cell receptor (TCR) and co-stimulatory molecules such as CD28, and through the interleukin-2 receptor (IL-2R). a | It is hypothesized that TReg cells have evolved to depend more heavily on signal transducer and activator of transcription 5 (STAT5)-dependent IL-2R signalling than on TCR and CD28 signalling compared with effector T cells. Dashed arrows and thick arrows indicate reduced and increased signalling, respectively. b | Effector T cells are poorly sensitive to in vitro IL-2 stimulation as measured by downstream STAT5 phosphorylation and they do not proliferate following in vivo treatment with low-dose IL-2. By contrast, TReg cells are highly sensitive to in vitro IL-2 stimulation and proliferate following low-dose IL-2 treatment in vivo. Data generated from cells obtained from patients with type 1 diabetes treated with low-dose IL-2 (Refs 82,85). Error bars indicate standard error of the mean. AP-1, activator protein 1; APC, antigen-presenting cell; IU, international unit; JAK, Janus kinase; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; NFAT, nuclear factor of activated T cells; PI3K, phosphoinositide 3-kinase.
Importantly, this greater sensitivity to IL-2 is not solely due to a higher level of expression of CD25 on TReg cells compared with effector T cells, as T cell blasts that have even higher levels of CD25 are less sensitive to IL-2-mediated activation than are TReg cells42. The exquisite sensitivity of TReg cells to IL-2 could thus also be due to IL-2R signalling specificity. Whereas the MAPK, PI3K–AKT and STAT5 pathways are all activated in effector T cells in response to antigen, co-stimulation and IL-2, TReg cells — in which high PTEN expression levels inhibit the PI3K-dependent signalling pathway43,44 — may be more dependent on IL-2–STAT5 signalling (Fig. 2).
In vivo experiments in mice confirmed these findings. At doses of IL-2 of approximately 20,000–50,000 IL-2 international units (IU) per day, TReg cell populations are expanded compared with those from control mice, and they express higher levels of activation markers and are more suppressive. At these doses of IL-2, neither clonal expansion nor activation of CD4+ or CD8+ effector T cells or of NK cells can be detected (see below).
IL-2 in pathophysiology
The inflammatory disease that develops in mice lacking IL-2 or functional IL-2R is characterized by colitis and the production of multiple autoantibodies14,15,16, but the specific effects can vary depending on the mouse strain. On the BALB/c background, the dominant phenotype is autoimmune haemolytic anaemia caused by erythrocyte-specific antibodies, whereas in C57BL/6 mice, the dominant phenotype is colitis. Over time, all Il2-knockout mouse strains produce autoantibodies that are specific for nucleoproteins and other self-antigens. Interestingly, the autoantibodies are produced even in germ-free Il2-knockout mice, whereas the colitis is largely abolished when these knockout mice are made germ free45,46. These data indicate that the defect in IL-2-dependent FOXP3+ TReg cells results in immune responses against self-antigens (leading to autoantibody production and 'true' autoimmunity), and perhaps also responses against commensal microorganisms in the gut (which may lead to colitis). In all of these models, the disease can be corrected by providing normal TReg cells.
Humans with rare mutations in CD25 also develop autoimmunity47. The disease caused by IL-2 or IL-2R deficiency is not as severe as that caused by mutations in FOXP3 in humans with immune dysregulation polyendocrinopathy enteropathy X-linked syndrome3 (IPEX syndrome) or in scurfy mice4. This might imply that some TReg cells are relatively IL-2 independent48,49. In line with this, it has been shown that IL-15 can substitute for IL-2 to support and expand TReg cell populations50.
In many individuals with autoimmune disease, IL-2 production is low compared with healthy individuals, and this could result in the instability and defective function of TReg cells. This is the case for autoimmune diseases such as type 1 diabetes51, rheumatoid arthritis52 and systemic lupus erythematosus (SLE)53. However, these findings are not typical of all patients with these diseases, and decreased IL-2 production or IL-2R expression is not considered to be a hallmark of most human autoimmune diseases.
Nonetheless, most autoimmune diseases involve a dysregulated balance between TReg cells and effector T cells, and thus, providing more IL-2-dependent signalling may help to correct the disease by increasing TReg cell number and function, even in the absence of a pre-existing TReg cell defect. This idea is highlighted by the therapeutic efficacy of IL-2 in mouse models in which there is not necessarily a measurable IL-2 or IL-2R defect. IL-2 and IL-2–antibody complex (IL-2c)54 have been administered in various experimental autoimmune diseases55,56,57,58,59,60 or other inflammatory settings61,62,63, and in all cases showed some (often remarkable) efficacy.
The ups and downs of IL-2 therapy
Therapeutic use of IL-2 in humans is often adjusted to weight (dose per kg) or body surface area (dose per m2). For ease of comparison of different studies, we have converted all dosing to a fixed dose corresponding to an adult of 70 kg and 1.8 m2 in the following discussion70.
Initial clinical development: why high doses? The field of IL-2 therapy started with the use of IL-2 purified from cell culture supernatant and gained pace after the cloning of IL-2 in 1983 (Ref. 64). Recombinant IL-2 was initially used at high doses, probably because of its short half-life, which was thought to explain the poor clinical response in the early clinical trials carried out with purified IL-2 (Refs 65,66). The first clinical trial reporting efficacy of IL-2 in human cancer67 used escalating doses of up to ~120 million IU (MIU), in three bolus infusions every 8 hours, to ensure that “serum levels were continuously maintained at concentration necessary to stimulate high affinity IL-2 receptors” (Ref. 70). Severe side effects were noted, including a generalized vascular leak syndrome (VLS), and increased serum creatinine and bilirubin levels (indicating kidney and liver damage). The maximum tolerated dose of IL-2 was found to be 42–50 MIU every 8 hours (126–150 MIU per day) and at this dosage, some complete clinical responses were observed in patients with renal cell carcinoma or melanoma68. Subsequent attempts to lower the IL-2 dose to reduce side effects resulted in decreased efficacy69.
Thus, the paradigm was established that (very) high doses of IL-2 were necessary to achieve a clinical response in patients with cancer, with the drawback of severe side effects.
High-dose IL-2 in cancer: lessons learnt. High doses of IL-2 were subsequently extensively used in patients with cancer. Importantly, they brought the first proof of concept that stimulation of immune responses could be a therapeutic modality in cancer. Large studies showed an ~15% response rate to IL-2 treatment, with 7–9% of patients with melanoma or renal cell carcinoma having complete and often long-lasting responses70. These results led to the approval of IL-2 therapy by the US Food and Drug Administration for renal cell carcinoma and metastatic melanoma in 1992 and 1998, respectively.
It is striking that more than 20 years later, the mechanisms of the successful immune responses induced by IL-2 therapy in patients with cancer are still not understood, and it is not clear why only some patients respond to high-dose IL-2. The discovery that IL-2 markedly expands TReg cell populations could, in part, explain treatment failures in some patients71,72,73,74.
The clinical efficacy of high-dose IL-2 in some patients with cancer is with the drawback of a high toxicity of the treatment. One of the main complications is VLS, which has recently been attributed to a direct effect of IL-2 on CD25-expressing endothelial cells75, and which leads to multi-organ dysfunction. Initial rates of mortality with high-dose IL-2 therapy were up to 4%, with deaths mostly occurring as a result of severe bacterial infections; these were later prevented by antibiotic therapy. Other common side effects of high-dose IL-2 are malaise, nausea and a flu-like syndrome, which are common manifestations of a cytokine storm, as is seen in settings such as acute infection76 and graft-versus-host disease (GVHD)77.
In summary, high-dose IL-2 has a poor safety profile but a robust efficacy in only a fraction of patients with a restricted set of cancers. High doses of IL-2 are still used in patients with these cancers, although the severe side effects and the inability to identify potential responders have severely restrained the use of high-dose IL-2 (Ref. 70).
The resurrection of IL-2: proof of concept. IL-2 has been available as an approved drug (Proleukin, Novartis; generic name aldesleukin) since 1992, and was thus available for clinical investigations. The effect of IL-2 on TReg cells was known since the mid-1990s, but it was more than 10 years before the first trials investigating the ability of IL-2 to promote TReg cell activity in humans with autoimmune and inflammatory diseases were initiated11,12. The common perception of IL-2 among clinicians was that of a very toxic drug that could be useful only in some late-stage cancers. In addition, IL-2 was considered to be a cytokine that primarily stimulates the effector arm of the immune response, and thus could aggravate autoimmune diseases. In fact, the Proleukin drug brochure specifically warns that “Proleukin has been associated with exacerbation of pre-existing or initial presentation of autoimmune disease and inflammatory disorders” (Ref. 121). Thus, most clinicians were not prepared to reconsider IL-2 as a therapy that could suppress immune responses in autoimmune diseases.
In 2006, researchers were searching for means by which to stimulate TReg cells in patients with hepatitis C virus-induced vasculitis (HCV-induced vasculitis). It had previously been shown that patients with HCV-induced vasculitis had a deficiency in the number of TReg cells78 and that the resolution of the vasculitis after treatment with antivirals or rituximab was associated with a normalization of TReg cell numbers79,80. IL-2 was thus suggested as a potential therapy for HCV-induced vasculitis, on the basis of a major TReg cell population expansion that had been observed in patients with cancer who were treated with high-dose IL-2 (Ref. 73). To avoid the severe side effects of high-dose IL-2 and also prevent, as far as possible, the activation of effector T cells, it was thought that using low-dose IL-2 could be the solution. Indeed, low-dose IL-2 had been well tolerated in other indications81 and the constitutively high levels of expression of CD25 on TReg cells could make them more sensitive than effector T cells to IL-2 (although this had not been definitively shown at the time).
Patients with HCV-induced vasculitis were treated with low-dose IL-2, with doses ranging from 1.5 MIU to 3 MIU per day. Patients received a cumulative dose of 50 MIU administered over 2 months11, which corresponds to one-third of the daily dose administered to some patients with cancer. The treatment was well tolerated, and there was a greater than twofold expansion of activated TReg cell populations with no detectable activation of effector T cells. A marked anti-inflammatory activity of IL-2 was also observed. Of note, a significant clinical improvement was seen in eight out of ten patients11. IL-2 was also shown to have beneficial effects for the treatment of chronic GVHD, an alloimmune inflammatory disease occurring after allogeneic haematopoietic stem cell transplantation (aHSCT)12. The results of these two trials showed that low-dose IL-2 could be a novel class of immunoregulatory drug that functions by inducing the expansion and/or activation of TReg cells. The door was thus opened for IL-2 therapy to be 'resurrected' into clinical use in the form of low-dose IL-2 to control autoimmunity and inflammation.
Clinical development of low-dose IL-2
The terminology 'low-dose IL-2' requires clarification. Of note, mouse models are not useful for defining low doses of IL-2 as applicable to humans because the IL-2 doses that stimulate TReg cells without stimulating effector T cells in mice (~20,000 IU) correspond to very high equivalent doses by weight for humans. In the cancer field, the term low-dose IL-2 has been used for doses of ~5 MIU per injection. However, these were given three times a day for long treatment periods, with the total amount of IL-2 administered to each patient being in excess of 100 MIU. In evaluating the safety and biological effects of low-dose IL-2, it is therefore important to consider both single and cumulative dosage. In the trials of low-dose IL-2 reviewed here, the single dose varies from 0.09 to 5.4 MIU and the cumulative dose ranges from 1.5 to 52.5 MIU, except for a few patients with chronic GVHD who received up to 320 MIU (see Supplementary information S1 (table)).
Safety. The safety of low-dose IL-2 was initially an important concern, but several trials have now established that low doses of IL-2 are well tolerated. Local reactions at injection sites and flu-like syndromes were the most frequent mild to moderate adverse events in patients or healthy volunteers receiving between 0.18 MIU and 3 MIU of IL-2 per day11,82,83,84 (Box 2; Fig. 3). Of note, in a double-blind, randomized, placebo-controlled trial, the total number of days on which adverse events were reported during the study was higher in the placebo group than in the IL-2-treated groups82. Together, these studies show that at doses that stimulate TReg cells, IL-2 is well tolerated.
At low doses, there are only mild to moderate adverse reactions to interleukin-2 (IL-2), including reactions of unknown cause at the site of injection and constitutional symptoms that are possibly triggered by cytokine release. The potential beneficial effects are indicated relative to paradigmatic diseases: atherosclerosis as an inflammatory disease; type 1 diabetes as a T cell-mediated autoimmune disease; systemic lupus erythematosus as an autoantibody-mediated autoimmune disease; and rheumatoid arthritis as an autoimmune inflammatory disease. pTReg, peripherally induced regulatory T; TFH, T follicular helper; TFR, T follicular regulatory; TH, T helper.
Main biological findings. The trials of low-dose IL-2 that have been reported so far have used different doses and different schedules of administration. However, the main conclusions are that IL-2 induces robust expansion of the TReg cell population, and that the maximum magnitude82 and duration85 of TReg cell population expansion are dose dependent. Some effects could be detected at an IL-2 dose as low as 0.18 MIU per day in healthy volunteers86. Although there are so far no descriptions of patients who do not respond to low-dose IL-2, one study has shown significant variations in the TReg cell response, with some patients who received the lowest dose of IL-2 (0.33 MIU per day) responding better than patients who received the highest dose (3 MIU per day)82. Of note, the minimum dose of IL-2 necessary to stimulate TReg cells has not yet been established, and may vary according to the patient and the disease.
The TReg cell response to IL-2 has mostly been studied in peripheral blood mononuclear cells (PBMCs). In one study, IL-2 induced a marked increase in the number of TReg cells that was accompanied by a marked decrease in the number of infiltrating CD8+ effector T cells in scalp biopsies of patients with autoimmune alopecia areata (see below); these patients markedly improved during treatment83. Thus, in line with observations made in animal models, the IL-2-dependent increase in the number of TReg cells that is mostly documented in peripheral blood is also seen at the site of the autoimmune manifestation.
The expansion of TReg cell populations is accompanied by a marked increase in their expression of activation markers, such as CD25, glucocorticoid-induced TNFR-related protein (GITR; also known as TNFRSF18) and cytotoxic T lymphocyte antigen 4 (CTLA4)85,87. In addition, the suppressive activity of these activated TReg cells is greater than that of control TReg cells11,12,88. Thus, we conclude that IL-2 induces the robust proliferation and activation of TReg cells, but its dose–response relationship in health and disease seems to be complex and needs to be investigated further to optimize IL-2 use.
A major proliferative response of CD8+CD25+FOXP3+ T cells to IL-2 has also been observed11,82. This increase in the number of CD8+ regulatory T cells is twofold to fivefold greater than the corresponding increase in the number of CD4+ TReg cells11,82; similar observations have been made in mice122 and macaques88. As CD8+CD25+FOXP3+ T cells are highly suppressive in vitro88,89,90,122, further studies are warranted to evaluate whether they have therapeutic effects in the context of disease.
NK cells also respond to low-dose IL-2, although much less so than TReg cells. In patients with chronic GVHD who received continuous treatment with IL-2, the number of blood NK cells doubled over the course of 8 weeks, whereas the number of TReg cells increased sixfold to eightfold12. In patients with type 1 diabetes, there was only a non-significant trend for a moderate increase in the number of NK cells at the highest dose of IL-2 used (3 MIU per day)82,85. In healthy volunteers, no effect of IL-2 on total NK cell number was reported. The non-cytotoxic CD56hi NK cells seemed to be most sensitive to IL-2 (Refs 11,86,87). Thus, low-dose IL-2 may in some cases expand NK cell populations, but in a daily and cumulative dose-dependent manner, and mostly for doses of more than 1 MIU per day.
In patients receiving continuous low-dose IL-2, a significant but asymptomatic eosinophilia peaked at 10% of white cells in the blood after 4 weeks of treatment, and subsequently declined12. In patients with HCV-induced vasculitis, there was a non-significant increase in the mean number of eosinophils after IL-2 treatment, with only some patients having increased values, and often not for all courses of IL-2 (Ref. 39). A modest but significant increase in the number of eosinophils was also seen in healthy volunteers who received IL-2. In summary, IL-2 does expand eosinophil populations, but in a daily and cumulative dose-dependent manner. At doses of less than or equal to 1 MIU per day, the effect seems to be minimal.
An intriguing and significant dose-dependent decrease in the number of CD19+ B cells, which also affected marginal zone B cells, has been reported in two trials of low-dose IL-2 (Refs 11,82), with a strong unexpected correlation between an increase in the number of TReg cells and a decrease in the number of B cells82. This effect could be linked to an inhibition of TFH cells and/or a stimulation of TFR cells. The clinical relevance of these findings remains to be clarified, particularly because B cell inhibition could be advantageous in the case of antibody-mediated autoimmune diseases for which B cell-depleting agents can be efficacious. Along these lines, three ongoing trials of low-dose IL-2 in patients with SLE have noted decreased levels of DNA-specific antibodies following IL-2 administration (see Supplementary information S1 (table)).
Immunomonitoring of patients with type 1 diabetes receiving increasing doses of low-dose IL-2 showed the dose-dependent induction of a regulatory milieu, as assessed by TReg cell to effector T cell ratios, TReg cell activation markers, plasma cytokine and chemokine levels, and transcriptomics85. This study also showed that low-dose IL-2 therapy did not induce IL-2-specific antibodies.
Altogether, doses of IL-2 below or equal to 1 MIU per day seem to specifically induce TReg cell expansion and activation, and a decrease in the number of B cells, both of which are desirable effects for the treatment of autoimmune diseases. At doses of more than 1 MIU per day, the effects on TReg cells are more marked, but some expansion of NK cell and eosinophil populations can be observed, which might be unwanted in some clinical settings.
Efficacy in human autoimmune and inflammatory diseases. Patients with HCV-induced vasculitis have a set of symptoms including fatigue, arthralgia, purpura, kidney involvement and neuropathy. In eight out of ten patients treated with low-dose IL-2, these symptoms progressively disappeared11. In most cases, clinical improvements started to be observed after the second or third course of IL-2 therapy11. In patients with alopecia areata, regrowth of the scalp and/or body hair was noted in all of the five patients treated with IL-2, and four had a regrowth of scalp hair83. Recently, early clinical results of low-dose IL-2 in patients with SLE that have been presented at meetings have reported marked improvement in clinical scores (see Supplementary information S1 (table)). In one patient with refractory SLE, a rapid induction of clinical remission was reported90.
In chronic GVHD, of the 23 patients who were evaluated — all of whom were refractory to systemic glucocorticoid therapy — 12 had a partial response to IL-2 and 11 had stable disease after IL-2 therapy12. In responding patients, extended therapy led to sustained clinical responses and a lowering of the corticosteroid doses administered, which is an indication of treatment efficacy. Low-dose IL-2 was also investigated for the prevention of GVHD in patients receiving aHSCT84. The IL-2 treatment not only reduced the frequency and severity of acute GVHD, but was also accompanied by a reduction in the number of infectious episodes, which are a major post-transplantation complication84.
Thus, although the dosing and timing of IL-2 administration may not have been optimal in these preliminary studies, they concordantly show some biological and clinical efficacy of low-dose IL-2 that correlates with the expansion and activation of TReg cell populations.
The promise of low-dose IL-2
By demonstrating the specificity of the effects of low-dose IL-2 on TReg cells and by reporting data that indicate a clinical benefit for patients, the preliminary studies discussed above have given optimism for further investigation. This has also been reinforced by mechanistic studies showing that, at least in theory, low-dose IL-2 should have therapeutic effects on both T cell-mediated and antibody-mediated autoimmune diseases.
The issue of dose and schedule. The current assumption is that the therapeutic potential of low-dose IL-2 is in large part linked to the stimulation of TReg cells, which are thus a surrogate marker of efficacy. Therefore, the question is how much TReg cell stimulation is required, and for how long, to have significant therapeutic effects in diseases that are often chronic in nature? The initial development of IL-2 therapy in patients with cancer was based on the assumption that constant detection of IL-2 in the serum was necessary for efficacy70, but it is not known whether constant TReg cell stimulation would be required in autoimmune diseases, or for how long.
By modelling the pharmacokinetics of TReg cells during and after a 5-day treatment of IL-2, we defined a 5-day induction course of 1 MIU of IL-2 per day as a scheme that will approximately double the percentage of TReg cells in PBMCs, to be followed by a fortnightly injection of 1 MIU of IL-2 as a maintenance treatment that should support an increased number of TReg cells at 20–60% above baseline values. This treatment modality is used in our current studies, and preliminary results from 36 patients in the TRANSREG clinical trial have confirmed these predictions. Importantly, we found that this treatment course is well tolerated in the long term (more than 6 months) and does not significantly modify the number of effector T cells, NK cells or eosinophils.
Further clinical trials will be required to better decipher the IL-2 dose–response relationship in terms of the specificity of TReg cell stimulation and treatment efficacy (Fig. 4). It should also be mentioned that continuous administration of IL-2 achieved by gene transfer is a possible treatment modality; this has been a convenient approach to use in experimental models91,92,93 but it would be less easy to adjust treatment over time if applied to humans.
The number of regulatory T (TReg) cells and their expression of activation markers are currently the best surrogate markers of the biological activity of interleukin-2 (IL-2). The efficacy of low-dose IL-2 treatment will probably depend on the extent and duration of these effects on TReg cells. Treatment efficacy can be characterized by the maximum effect gained (Emax) and the overall effect over time, which is defined by the area under the curve (AUC; compared with baseline). The dose and schedule of IL-2 administration will have a major influence on these parameters, as well as on the specificity of the effect for TReg cells as opposed to effector T cells, natural killer (NK) cells and eosinophils. a | Repeated courses of IL-2 (indicated by the red arrows) at defined intervals11,83,90 will produce an increase in the number of TReg cells at the end of each course, followed by a decrease in their number to a level that depends on the dose administered85 and the timing of the next course11. b | Daily continuous administration of IL-2 will produce the greatest overall effects (highest AUC), but with the side effect of some expansion of NK cell, effector T cell and eosinophil populations12. c | A short induction course followed by maintenance treatment with IL-2 will enable a desired Emax to be reached, and then a desired AUC to be maintained by repeated single injections, the timing of which will determine the long-term AUC. d | Repeated single administration of IL-2 will provide transient boosts to TReg cell numbers. e | Personalized biomarker-defined administration will be the ultimate treatment modality when (or if) biomarkers of efficacy are identified. At present (in the absence of an ideal personalized biomarker-controlled administration schedule), the induction course followed by maintenance treatment with IL-2, or repeated courses of IL-2, might be appropriate for settings in which the disease is moderate and there is a need for long-term support of TReg cells (for example, in patients with recently diagnosed type 1 diabetes). In more severe or more inflammatory settings, daily continuous administration of IL-2 might be favourable (for example, in patients with chronic graft-versus-host disease). Finally, repeated single administration of IL-2 might be more appropriate for treatments that aim to support the suppressive activity of TReg cells over long periods of time in conditions in which this activity may be defective owing to IL-2 deprivation (for example, for the prevention of type 1 diabetes).
Immunoregulation without immunosuppression. The main complications of immunosuppression are related to infection. As there is normally a balance between TReg cells and effector T cells, and because TReg cells also control immune responses to infection that are mediated by effector T cells, the question thus arises as to whether protective antimicrobial immunity would be compromised when enhancing TReg cell function in a non-antigen-specific manner. Preclinical and clinical data available so far indicate that this is not the case. The administration of an IL-2-expressing adeno-associated virus vector in mice maintained IL-2 production and the associated increase in the number of TReg cells for more than a year93. Under such conditions, which permanently increase the proportion of TReg cells to 150–200% of baseline values, no effects were observed on mouse lifespan, clinical signs or tissue histology. Furthermore, when challenged with an influenza virus vaccine or a lethal dose of a mouse-adapted influenza virus, mice receiving long-term administration of IL-2 mounted normal protective immune responses93.
In patients with chronic HCV infection, no increase in viral load was observed during IL-2 treatment despite increased TReg cell numbers, and there was even a small but significant decrease in the viral load at the end of the follow-up period11. In the GVHD-prevention trial, the recovery of in vitro immune responses against recall antigens was identical in the IL-2-treated and control groups, and, importantly, a marked decrease in the number of infectious episodes was observed in the treated group as compared with controls84. Nevertheless, given the effects of IL-2 on TFH cells and TFR cells, and the striking decrease in the number of B cells that has been reported in some trials of IL-2 (Refs 11,82), further studies of humoral responses in individuals treated with IL-2 are warranted.
In the trial of low-dose IL-2 therapy for chronic GVHD, three patients had bacterial infections of grade 3 or higher12, but these findings are difficult to interpret in light of the other immunosuppressive drugs or corticosteroids that these patients received. Of note, in a trial investigating the adoptive transfer of ex vivo-expanded TReg cells in children with type 1 diabetes, one patient contracted an influenza virus infection immediately after the TReg cell infusion. The infection resolved normally within a few days, indicating that increased TReg cell number did not induce systemic immune suppression7.
The apparent preservation of a normal immune response to infection during IL-2 therapy is, in fact, not surprising. Inflammation is controlled by TReg cells but it also controls TReg cells94. At high levels of inflammation, TReg cells become less efficient95. It is thus expected that a moderate increase in the number of TReg cells or their functionality should not affect effector immune responses that are triggered by highly inflammatory events, such as infection or vaccination, as the inflammation would be expected to constrain the TReg cell response.
IL-2 and inflammation. On the one hand, TReg cells tend to lose their functional capacity in highly inflammatory environments94. They might even become unstable, losing FOXP3 expression and converting to a phenotype that is more characteristic of effector CD4+ T cells (these converted cells have been termed 'ex-TReg cells'), although such a process is much debated96. Different mechanisms can regulate the susceptibility of TReg cells to inflammation-induced FOXP3 destabilization: FOXP3 expression is downregulated by polyubiquitylation97 mediated by the E3 ubiquitin ligase STUB1, which is induced by pro-inflammatory cytokines and lipopolysaccharides98; PIM1 kinase phosphorylates a serine residue of human FOXP3, thereby impairing its function99; and binding of methyl CpG-binding protein 2 (MeCP2) to FOXP3 stabilizes its expression100.
On the other hand, TReg cells suppress inflammation by multiple mechanisms, including reducing co-stimulation to activate effector T cells, consuming IL-2 and secreting immunosuppressive cytokines such as IL-10. The anti-inflammatory effects of TReg cells have been observed in various models of inflammatory diseases in mice. In a model of atherosclerosis that spontaneously develops in low-density lipoprotein receptor-knockout mice, TReg cell depletion aggravated atherosclerosis and TReg cell repletion improved it61. In line with these observations, treatment with IL-2 also improved atherosclerotic lesions101,102. Recent reports confirmed that TReg cells can improve other inflammatory conditions, such as acute lung injury103, muscular dystrophy63 or beryllium-induced granulomatous inflammation62. In the muscular dystrophy model, treatment with IL-2c increased the number of TReg cells and the IL-10 concentration in muscle, resulting in decreased myofibre injury. In humans treated with IL-2, the analysis of the entire transcriptome of PBMCs suggested a major anti-inflammatory role of low-dose IL-2 (Ref. 11).
These results warrant the investigation of low-dose IL-2 in inflammatory diseases or conditions that are not caused by autoimmune or alloimmune reactions (Fig. 3).
IL-2 in other indications. TReg cells have a role in controlling allergy, as indicated by the presence of this symptom in the IPEX syndrome. IL-2c has been shown to improve an experimental form of allergy in mice104. Thus, IL-2 also has some potential for the treatment of allergy. However, the dose-dependent eosinophilia that can be induced by IL-2 should be carefully considered in terms of the application of this therapy to asthma, in which the harmful role of eosinophils is still debated105. Furthermore, ILC2s can respond to IL-2 by producing IL-13, which is detrimental in allergy and asthma106. IL-2 has already been evaluated, with some success, in the fields of HSCT12,84 and solid organ transplantation107.
Ageing of the immune system is associated with abnormalities of the T cell repertoire, with the appearance of oligoclonal expansions108. Injection of TReg cells into old mice or the treatment of these mice with IL-2 prevented such oligoclonal T cell expansions109. The role of IL-2 in preserving T cell homeostasis during ageing should be more thoroughly investigated.
Second-generation IL-2 and combination therapies. Fusions of IL-2 with carrier proteins, such as the Fc domain of IgG, have improved its short half-life110. In addition, several mutant forms of IL-2 have been produced in attempts to alter its ability to bind to different components of the IL-2R, and thus to change its range of activity. One of the earliest such mutant forms of IL-2 was designed to reduce binding to the CD122–CD132 IL-2R dimer (to reduce activation of NK cells and cytokine-mediated toxicity), while preserving its ability to bind to CD25 and hence the trimeric high-affinity IL-2R111. This mutant IL-2 protein retained the ability to activate T cells and mediate antitumour effects, but its effect on TReg cells has not yet been reported. A different IL-2 mutant has been produced that shows increased binding to CD122 and thus has an increased ability to activate CD8+ T cells and NK cells112. Complexes of specific monoclonal antibodies bound to IL-2 have modified the interaction with IL-2R complexes. Depending on the antibody-binding site on IL-2, the IL-2c preferentially activates cells expressing high levels of CD122, such as memory CD8+ T cells and NK cells, or CD25-expressing cells such as TReg cells54,113.
Although this field is extremely active and worth pursuing, it should be kept in mind that modified IL-2 or IL-2c with preferential activity for TReg cells has not yet been evaluated in patients. It will be many years before an optimized TReg cell-focused form of IL-2 is at the same stage of development as 'plain' IL-2, with its 30 years of clinical use. Thus, it is reasonable to propose that clinical development of plain IL-2 should be pursued without waiting for the promises of more effective variants; also, the experience gained with plain IL-2 will help to develop second-generation versions of IL-2.
Combination therapy is often superior to single therapy for complex diseases. High-dose IL-2 is already being tested in combination with checkpoint inhibitors for the immunotherapy of cancer114. Similarly, combinations of low-dose IL-2 with other immune modulators will be worth evaluating in autoimmune diseases. For example, combining low-dose IL-2 with antibodies targeting inflammatory cytokines such as IL-1, IL-6, IL-17 or IL-23 could prove to have synergistic effects in controlling immune-triggered inflammation.
Finally, the possibility to combine the use of IL-2 with antigens to induce specific tolerance is extremely attractive and should be explored.
Conclusions and perspectives
Independent clinical trials have now shown the safety of low-dose IL-2, at least for treatment lengths ranging from several months to a year, which overcomes the most frequent barrier in the development of novel therapies. Moreover, these trials have provided preliminary indications of significant biological and clinical efficacy. Without waiting for a comprehensive understanding of its mechanisms of action, the promises of low-dose IL-2 therapy already warrant broad clinical investigations. These should be aimed at exploring the large field of potential target diseases, but also at transforming current indications of efficacy into proof of efficacy that can only be obtained from state of the art, double-blind, placebo-controlled randomized trials. Comprehensive ancillary studies embedded in these clinical trials should help to harness IL-2 as the next revolution in immunotherapy — immunoregulation without immunosuppression.
References
Sakaguchi, S., Powrie, F. & Ransohoff, R. M. Re-establishing immunological self-tolerance in autoimmune disease. Nature Med. 18, 54–58 (2012).
Shevach, E. M. & Thornton, A. M. tTregs, pTregs, and iTregs: similarities and differences. Immunol. Rev. 259, 88–102 (2014).
Moraes-Vasconcelos, D., Costa-Carvalho, B. T., Torgerson, T. R. & Ochs, H. D. Primary immune deficiency disorders presenting as autoimmune diseases: IPEX and APECED. J. Clin. Immunol. 28 (Suppl. 1), 11–19 (2008).
Godfrey, V., Wilkinson, J. & Russell, L. X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am. J. Pathol. 138, 1379–1387 (1991).
Noack, M. & Miossec, P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 13, 668–677 (2014).
Kim, J. M., Rasmussen, J. P. & Rudensky, A. Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature Immunol. 8, 191–197 (2007). T Reg cell depletion in otherwise healthy individuals triggers brisk autoimmune responses, underscoring the permanent suppression of potentially harmful effector T cells that occurs during homeostasis.
Marek-Trzonkowska, N. et al. Administration of CD4+CD25highCD127− regulatory T cells preserves β-cell function in type 1 diabetes in children. Diabetes Care 35, 1817–1820 (2012).
Malek, T. R. The biology of interleukin-2. Annu. Rev. Immunol. 26, 453–479 (2008).
Boyman, O. & Sprent, J. The role of interleukin-2 during homeostasis and activation of the immune system. Nature Rev. Immunol. 12, 180–190 (2012).
Liao, W., Lin, J. X. & Leonard, W. J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 38, 13–25 (2013).
Saadoun, D. et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N. Engl. J. Med. 365, 2067–2077 (2011). The first clinical trial showing that low-dose IL-2 is safe and clinically efficacious, and that it induces T Reg cells, in an autoimmune disease.
Koreth, J. et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N. Engl. J. Med. 365, 2055–2066 (2011). The first clinical trial showing that low-dose IL-2 is safe and clinically efficacious, and that it induces T Reg cells, in an alloimmune inflammatory disease.
Robb, R. J. Interleukin 2: the molecule and its function. Immunol. Today 5, 203–209 (1984).
Sadlack, B. et al. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75, 253–261 (1993). The first evidence that IL-2 is involved in preventing autoimmune diseases.
Suzuki, H. et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science 268, 1472–1476 (1995).
Willerford, D. M. et al. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity 3, 521–530 (1995).
Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155, 1151–1164 (1995). The discovery of T Reg cells and their role in preventing autoimmune diseases.
Malek, T. R., Yu, A., Vincek, V., Scibelli, P. & Kong, L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice. Implications for the nonredundant function of IL-2. Immunity 17, 167–178 (2002).
Vang, K. B. et al. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J. Immunol. 181, 3285–3290 (2008).
Fontenot, J. D., Rasmussen, J. P., Gavin, M. A. & Rudensky, A. Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nature Immunol. 6, 1142–1151 (2005).
Barron, L. et al. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J. Immunol. 185, 6426–6430 (2010).
Feng, Y. et al. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell 158, 749–763 (2014).
Ohkura, N. et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 37, 785–799 (2012).
Williams, M. A., Tyznik, A. J. & Bevan, M. J. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 441, 890–893 (2006).
Kalia, V. et al. Prolonged interleukin-2Rα expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity 32, 91–103 (2010).
Pipkin, M. E. et al. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity 32, 79–90 (2010).
Setoguchi, R., Hori, S., Takahashi, T. & Sakaguchi, S. Homeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J. Exp. Med. 201, 723–735 (2005).
Bilate, A. M. & Lafaille, J. J. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu. Rev. Immunol. 30, 733–758 (2012).
Cote-Sierra, J. et al. Interleukin 2 plays a central role in TH2 differentiation. Proc. Natl Acad. Sci. USA 101, 3880–3885 (2004).
Liao, W. et al. Opposing actions of IL-2 and IL-21 on Th9 differentiation correlate with their differential regulation of BCL6 expression. Proc. Natl Acad. Sci. USA 111, 3508–3513 (2014).
Liao, W., Lin, J.-X., Wang, L., Li, P. & Leonard, W. J. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nature Immunol. 12, 551–559 (2011).
Laurence, A. et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 26, 371–381 (2007).
Ballesteros-Tato, A. et al. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity 36, 847–856 (2012).
Linterman, M. A. et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nature Med. 17, 975–982 (2011).
Chung, Y. et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nature Med. 17, 983–988 (2011).
Wollenberg, I. et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J. Immunol. 187, 4553–4560 (2011).
Neill, D. R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010).
Roediger, B. et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nature Immunol. 14, 564–573 (2013).
Van Gool, F. et al. Interleukin-5 producing group 2 innate lymphoid cells (ILC2) control eosinophilia induced by interleukin-2 therapy. Blood 124, 3572–3576 (2014).
Ribot, J. C., Ribeiro, S. T., Correia, D. V., Sousa, A. E. & Silva-Santos, B. Human γδ thymocytes are functionally immature and differentiate into cytotoxic type 1 effector T cells upon IL-2/IL-15 signaling. J. Immunol. 192, 2237–2243 (2014).
Yu, A., Zhu, L., Altman, N. H. & Malek, T. R. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity 30, 204–217 (2009). The molecular explanation for the specific effects of low-dose IL-2 on T Reg cells.
Yu, A. et al. Selective IL-2 responsiveness of regulatory T cells through multiple intrinsic mechanisms support the use of low-dose IL-2 therapy in type-1 diabetes. Diabetes http://dx.doi.org/10.2337/db14-1322 (2015).
Bensinger, S. J. et al. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J. Immunol. 172, 5287–5296 (2004).
Walsh, P. T. et al. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J. Clin. Invest. 116, 2521–2531 (2006).
Contractor, N. V. et al. Lymphoid hyperplasia, autoimmunity, and compromised intestinal intraepithelial lymphocyte development in colitis-free gnotobiotic IL-2-deficient mice. J. Immunol. 160, 385–394 (1998).
Schultz, M. et al. IL-2-deficient mice raised under germfree conditions develop delayed mild focal intestinal inflammation. Am. J. Physiol. 276, G1461–G1472 (1999).
Sharfe, N., Dadi, H. K., Shahar, M. & Roifman, C. M. Human immune disorder arising from mutation of the α chain of the interleukin-2 receptor. Proc. Natl Acad. Sci. USA 94, 3168–3171 (1997).
Smigiel, K. S. et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J. Exp. Med. 211, 121–136 (2014).
Gratz, I. K. et al. Cutting edge: memory regulatory T cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J. Immunol. 190, 4483–4487 (2013).
Raynor, J. et al. IL-15 fosters age-driven regulatory T cell accrual in the face of declining IL-2 levels. T Cell Biol. 4, 161 (2013).
Zier, K. S., Leo, M. M., Spielman, R. S. & Baker, L. Decreased synthesis of interleukin-2 (IL-2) in insulin-dependent diabetes mellitus. Diabetes 33, 552–555 (1984).
Kitas, G. D., Salmon, M., Farr, M., Gaston, J. S. & Bacon, P. A. Deficient interleukin 2 production in rheumatoid arthritis: association with active disease and systemic complications. Clin. Exp. Immunol. 73, 242–249 (1988).
Lieberman, L. A. & Tsokos, G. C. The IL-2 defect in systemic lupus erythematosus disease has an expansive effect on host immunity. J. Biomed. Biotechnol. 2010, 740619 (2010).
Boyman, O., Kovar, M., Rubinstein, M. P., Surh, C. D. & Sprent, J. Selective stimulation of T cell subsets with antibody–cytokine immune complexes. Science 311, 1924–1927 (2006). The first report of the properties of IL-2–antibody complexes.
Rouse, M., Nagarkatti, M. & Nagarkatti, P. S. The role of IL-2 in the activation and expansion of regulatory T-cells and the development of experimental autoimmune encephalomyelitis. Immunobiology 218, 674–682 (2013).
Hao, J. et al. Interleukin-2/interleukin-2 antibody therapy induces target organ natural killer cells that inhibit central nervous system inflammation. Ann. Neurol. 69, 721–734 (2011).
Grinberg-Bleyer, Y. et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J. Exp. Med. 207, 1871–1878 (2010).
Mizui, M. et al. IL-2 protects lupus-prone mice from multiple end-organ damage by limiting CD4−CD8− IL-17-producing T cells. J. Immunol. 193, 2168–2177 (2014).
Tang, Q. et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 28, 687–697 (2008).
Liu, R. et al. Expansion of regulatory T cells via IL-2/anti-IL-2 mAb complexes suppresses experimental myasthenia. Eur. J. Immunol. 40, 1577–1589 (2010).
Ait-Oufella, H. et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nature Med. 12, 178–180 (2006).
Mack, D. G. et al. Regulatory T cells modulate granulomatous inflammation in an HLA-DP2 transgenic murine model of beryllium-induced disease. Proc. Natl Acad. Sci. USA 111, 8553–8558 (2014).
Villalta, S. A. et al. Regulatory T cells suppress muscle inflammation and injury in muscular dystrophy. Sci. Transl. Med. 6, 258ra142 (2014).
Taniguchi, T. et al. Structure and expression of a cloned cDNA for human interleukin-2. Nature 302, 305–310 (1983).
Bindon, C. et al. Clearance rates and systemic effects of intravenously administered interleukin 2 (IL-2) containing preparations in human subjects. Br. J. Cancer 47, 123–133 (1983).
Lotze, M. T., Frana, L. W., Sharrow, S. O., Robb, R. J. & Rosenberg, S. A. In vivo administration of purified human interleukin 2. I. Half-life and immunologic effects of the Jurkat cell line-derived interleukin 2. J. Immunol. 134, 157–166 (1985).
Rosenberg, S. A. et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med. 313, 1485–1492 (1985).
Rosenberg, S. A. et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 271, 907–913 (1994). A large study of high-dose IL-2 in patients with cancer.
Yang, J. C. et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J. Clin. Oncol. 21, 3127–3132 (2003).
Rosenberg, S. A. IL-2: the first effective immunotherapy for human cancer. J. Immunol. 192, 5451–5458 (2014).
Ahmadzadeh, M. & Rosenberg, S. A. IL-2 administration increases CD4+ CD25hi Foxp3+ regulatory T cells in cancer patients. Blood 107, 2409–2414 (2006).
Zhang, H. et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nature Med. 11, 1238–1243 (2005).
Lemoine, F. M. et al. Massive expansion of regulatory T-cells following interleukin 2 treatment during a Phase I–II dendritic cell-based immunotherapy of metastatic renal cancer. Int. J. Oncol. 35, 569–581 (2009).
Weiss, L. et al. In vivo expansion of naive and activated CD4+CD25+FOXP3+ regulatory T cell populations in interleukin-2-treated HIV patients. Proc. Natl Acad. Sci. USA 107, 10632–10637 (2010).
Krieg, C., Letourneau, S., Pantaleo, G. & Boyman, O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc. Natl Acad. Sci. USA 107, 11906–11911 (2010).
London, N. R. et al. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci. Transl. Med. 2, 23ra19 (2010).
Ferrara, J. L. Cytokine dysregulation as a mechanism of graft versus host disease. Curr. Opin. Immunol. 5, 794–799 (1993).
Boyer, O. et al. CD4+CD25+ regulatory T-cell deficiency in patients with hepatitis C-mixed cryoglobulinemia vasculitis. Blood 103, 3428–3430 (2004).
Saadoun, D. et al. Restoration of peripheral immune homeostasis after rituximab in mixed cryoglobulinemia vasculitis. Blood 111, 5334–5341 (2008).
Landau, D.-A. et al. Correlation of clinical and virologic responses to antiviral treatment and regulatory T cell evolution in patients with hepatitis C virus-induced mixed cryoglobulinemia vasculitis. Arthritis Rheum. 58, 2897–2907 (2008).
Alric, L. et al. Pilot study of interferon-α-ribavirin-interleukin-2 for treatment of nonresponder patients with severe liver disease infected by hepatitis C virus genotype 1. J. Viral Hepat. 13, 139–144 (2006).
Hartemann, A. et al. Low-dose interleukin 2 in patients with type 1 diabetes: a Phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 1, 295–305 (2013).
Castela, E. et al. Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol. 150, 748–751 (2014).
Kennedy-Nasser, A. A. et al. Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin. Cancer Res. 20, 2215–2225 (2014).
Rosenzwajg, M. et al. Low-dose interleukin-2 fosters a dose-dependent regulatory-tuned milieu in T1D patients. J. Autoimmun. 58, 48–58 (2015).
Ito, S. et al. Ultra-low dose interleukin-2 promotes immune-modulating function of regulatory T cells and natural killer cells in healthy volunteers. Mol. Ther. J. Am. Soc. Gene Ther. 22, 1388–1395 (2014).
Matsuoka, K. et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci. Transl. Med. 5, 179ra43 (2013).
Aoyama, A. et al. Low-dose IL-2 for in vivo expansion of CD4+ and CD8+ regulatory T cells in nonhuman primates. Am. J. Transpl. 12, 2532–2537 (2012).
Chaput, N. et al. Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut 58, 520–529 (2009).
Humrich, J. Y. et al. Rapid induction of clinical remission by low-dose interleukin-2 in a patient with refractory SLE. Ann. Rheum. Dis. 74, 791–792 (2015).
Gutierrez-Ramos, J. C., Andreu, J. L., Revilla, Y., Vinuela, E. & Martinez, C. Recovery from autoimmunity of MRL/lpr mice after infection with an interleukin-2/vaccinia recombinant virus. Nature 346, 271–274 (1990).
Goudy, K. S. et al. Inducible adeno-associated virus-mediated IL-2 gene therapy prevents autoimmune diabetes. J. Immunol. 186, 3779–3786 (2011).
Churlaud, G. et al. Sustained stimulation and expansion of Tregs by IL-2 control autoimmunity without impairing immune responses to infection, vaccination and cancer. Clin. Immunol. 151, 114–126 (2014).
Yamaguchi, T., Wing, J. B. & Sakaguchi, S. Two modes of immune suppression by Foxp3+ regulatory T cells under inflammatory or non-inflammatory conditions. Semin. Immunol. 23, 424–430 (2011).
Billiard, F. et al. Regulatory and effector T cell activation levels are prime determinants of in vivo immune regulation. J. Immunol. 177, 2167–2174 (2006).
Sakaguchi, S., Vignali, D. A. A., Rudensky, A. Y., Niec, R. E. & Waldmann, H. The plasticity and stability of regulatory T cells. Nature Rev. Immunol. 13, 461–467 (2013).
Van Loosdregt, J. et al. Stabilization of the transcription factor Foxp3 by the deubiquitinase USP7 increases Treg-cell-suppressive capacity. Immunity 39, 259–271 (2013).
Chen, Z. et al. The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity 39, 272–285 (2013).
Li, Z. et al. PIM1 kinase phosphorylates the human transcription factor FOXP3 at serine 422 to negatively regulate its activity under inflammation. J. Biol. Chem. 289, 26872–26881 (2014).
Li, C. et al. MeCP2 enforces Foxp3 expression to promote regulatory T cells' resilience to inflammation. Proc. Natl Acad. Sci. USA 111, E2807–E2816 (2014).
Dietrich, T. et al. Local delivery of IL-2 reduces atherosclerosis via expansion of regulatory T cells. Atherosclerosis 220, 329–336 (2012).
Dinh, T. N. et al. Cytokine therapy with interleukin-2/anti-interleukin-2 monoclonal antibody complexes expands CD4+CD25+Foxp3+ regulatory T cells and attenuates development and progression of atherosclerosis. Circulation 126, 1256–1266 (2012).
D'Alessio, F. R. et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J. Clin. Invest. 119, 2898–2913 (2009).
Wilson, M. S. et al. Suppression of murine allergic airway disease by IL-2: anti-IL-2 monoclonal antibody-induced regulatory T cells. J. Immunol. 181, 6942–6954 (2008).
Rosenberg, H. F., Dyer, K. D. & Foster, P. S. Eosinophils: changing perspectives in health and disease. Nature Rev. Immunol. 13, 9–22 (2013).
Oliphant, C. J. et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4+ T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 41, 283–295 (2014).
Zheng, X. X. et al. Favorably tipping the balance between cytopathic and regulatory T cells to create transplantation tolerance. Immunity 19, 503–514 (2003).
Blackman, M. A. & Woodland, D. L. The narrowing of the CD8 T cell repertoire in old age. Curr. Opin. Immunol. 23, 537–542 (2011).
Thomas-Vaslin, V. et al. in Immunosuppression — Role in Health and Diseases. (eds Kapur, S. & Portela, M. B.) 125–147 (Intech, 2012).
Harvill, E. T. & Morrison, S. L. An IgG3-IL2 fusion protein activates complement, binds Fcγ RI, generates LAK activity and shows enhanced binding to the high affinity IL-2R. Immunotechnol. Int. J. Immunol. Eng. 1, 95–105 (1995).
Shanafelt, A. B. et al. A T-cell-selective interleukin 2 mutein exhibits potent antitumor activity and is well tolerated in vivo. Nature Biotech. 18, 1197–1202 (2000).
Levin, A. M. et al. Exploiting a natural conformational switch to engineer an interleukin-2 'superkine'. Nature 484, 529–533 (2012).
Rosalia, R. A., Arenas-Ramirez, N., Bouchaud, G., Raeber, M. E. & Boyman, O. Use of enhanced interleukin-2 formulations for improved immunotherapy against cancer. Curr. Opin. Chem. Biol. 23, 39–46 (2014).
Kaufman, H. L. et al. The society for immunotherapy of cancer consensus statement on tumour immunotherapy for the treatment of cutaneous melanoma. Nature Rev. Clin. Oncol. 10, 588–598 (2013).
Kasakura, S. & Lowenstein, L. A factor stimulating DNA synthesis derived from the medium of leukocyte cultures. Nature 208, 794–795 (1965).
Gordon, J. & MacLean, L. D. A lymphocyte-stimulating factor produced in vitro. Nature 208, 795–796 (1965).
Morgan, D. A., Ruscetti, F. W. & Gallo, R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science 193, 1007–1008 (1976). The first evidence of a T cell growth factor (IL-2) produced by activated T cells.
Gillis, S., Baker, P. E., Ruscetti, F. W. & Smith, K. A. Long-term culture of human antigen-specific cytotoxic T-cell lines. J. Exp. Med. 148, 1093–1098 (1978).
Smith, K. A. Interleukin-2: inception, impact, and implications. Science 240, 1169–1176 (1988).
Edwards, I. R. & Aronson, J. K. Adverse drug reactions: definitions, diagnosis, and management. Lancet 356, 1255–1259 (2000).
Prometheus Laboratories. Proleukin® (aldesleukin). [online], (2012).
Churlaud, G. et al. Human and mouse CD8+CD25+FOXP3+ regulatory T cells at steady state and during interleukin-2 therapy. Front. Immunol. http://dx.doi.org/10.3389/fimmu.2015.00171 (2015).
Acknowledgements
The work of D.K. is funded by French state funds within the Investissements d'Avenir programme (ANR-11-IDEX-0004-02; LabEx Transimmunom); the European Research Council Advanced Grant (ERC-2012-AdG, TRiPoD, Agreement number 322856); the Assistance Publique – Hopitaux de Paris, France; the Sorbonne University, Pierre and Marie Curie Medical School, Paris, France; the Institut National de la Santé et de la Recherche Médicale (INSERM); and Le Centre National de la Recherche Scientifique (CNRS).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
D.K. is an inventor of a patent application claiming low-dose IL-2 for therapy of autoimmune diseases, owned by his academic institution, and licensed to ILTOO Pharma, which he advises and in which he holds shares. A.K.A. declares no competing interests.
Related links
DATABASES
Supplementary information
Supplementary information S1 (table)
Main clinical and biological findings from high- to low-dose IL-2 clinical trials (PDF 269 kb)
Glossary
- T follicular regulatory cells
-
(TFR cells). Cells that are derived from thymus-derived regulatory T cells and share phenotypic characteristics with T follicular helper (TFH) cells, including the expression of the B cell follicle-homing receptor CXC-chemokine receptor 5. TFH cells control germinal centre B cells undergoing somatic hypermutation and selection that results in antibody affinity maturation. TFR cells reduce TFH cell and germinal centre B cell numbers.
- Type 2 innate lymphoid cells
-
(ILC2s). ILCs are rare cells from the lymphoid lineage — comprising the ILC1, ILC2 and ILC3 subsets — that produce many of the same cytokines as T cells but lack T cell antigen receptors. They have diverse roles in immune responses and inflammation. ILC2s produce type 2 cytokines, such as interleukin-5 (IL-5) and IL-13, and have key roles in anthelminthic responses and allergic lung inflammation.
- IL-2 international units
-
(IL-2 IU). The interleukin-2 (IL-2) IU is a biological activity determined by the ability to support the growth of an IL-2-dependent cell line. In practice, as the manufacture of IL-2 is standardized, 1.1 mg of IL-2 corresponds to 18 million IU.
- Immune dysregulation polyendocrinopathy enteropathy X-linked syndrome
-
(IPEX syndrome). This rare genetic autoimmune disease is caused by mutations in the FOXP3 gene (which encodes forkhead box P3). Affected males present with early-onset severe enteropathy that is usually accompanied by insulin-dependent diabetes (type 1). Other autoimmune manifestations include hypothyroidism, anaemia, thrombocytopenia and neutropenia. Increased serum IgE levels and dermatitis are often also present.
- IL-2–antibody complex
-
(IL-2c). A complex of interleukin-2 (IL-2) and IL-2-specific antibody that has a prolonged half-life compared with IL-2. Its biological activity depends on the antibody. Some complexes preferentially stimulate effector T cells, whereas others preferentially stimulate regulatory T cells.
- Vascular leak syndrome
-
(VLS). VLS (also known as capillary leak syndrome, systemic capillary leak syndrome or Clarkson disease) is characterized by a leakage of fluid from the circulatory system into the interstitial space, resulting in hypotension, haemoconcentration and hypoalbuminaemia.
- Cytokine storm
-
Also known as hypercytokinaemia. The systemic expression of a vigorous immune response resulting in the release of inflammatory mediators into the bloodstream, including cytokines, oxygen free radicals and coagulation factors. The primary symptoms of a cytokine storm are high fever, swelling and redness caused by vascular leak, extreme fatigue and nausea.
- Graft-versus-host disease
-
(GVHD). A common complication of allogeneic stem cell transplantation, in which immune cells from the graft recognize the recipient cells as foreign and attack them. The main target tissues are the liver, skin and gastrointestinal tract. It can be acute (within 100 days of transplantation) and life-threatening, or chronic.
- Hepatitis C virus-induced vasculitis
-
(HCV-induced vasculitis). 10–15% of patients with chronic HCV infection develop systemic cryoglobulinaemic vasculitis. Cryoglobulins are cold-insoluble immune complexes containing rheumatoid factor and polyclonal IgG that deposit on vascular endothelium, causing vasculitis in organs such as the skin and kidneys, and in peripheral nerves.
- Adverse events
-
Medical occurrences that are temporally (but not necessarily causally) associated with the use of a medicinal product. The severity of adverse events is graded from 1 to 4, with the grades representing mild, moderate, severe and potentially life-threatening events, respectively. Any adverse event that causes death, is life threatening, requires hospitalization, or results in persistent or significant disability or incapacity is considered a serious adverse event.
- Alopecia areata
-
A prevalent autoimmune disease (affecting 1.7% of the general population) that leads to hair loss on the scalp and other areas of the body. Infiltration of CD4+ and CD8+ T cells around hair follicles is associated with the condition.
- TRANSREG clinical trial
-
An open-label Phase II clinical trial investigating the stimulation of regulatory T cells by low-dose interleukin-2 in 11 autoimmune diseases: rheumatoid arthritis, ankylosing spondylitis, systemic lupus erythematosus, psoriasis, Behcet disease, Wegener granulomatosis, Takayasu disease, Crohn disease, ulcerative colitis, autoimmune hepatitis and sclerosing cholangitis (ClinicalTrials.gov identifier: NCT01988506).
Rights and permissions
About this article
Cite this article
Klatzmann, D., Abbas, A. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol 15, 283–294 (2015). https://doi.org/10.1038/nri3823
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3823
This article is cited by
-
Identifying the sensor elements of regulatory T cells in atherosclerosis
Nature Cardiovascular Research (2024)
-
Efficacy, Safety and the Lymphocyte Subset Changes of Low-Dose IL-2 in Patients with Autoimmune Rheumatic Diseases: A Systematic Review and Meta-Analysis
Rheumatology and Therapy (2024)
-
Molecular Engineering of Interleukin-2 for Enhanced Therapeutic Activity in Autoimmune Diseases
BioDrugs (2024)
-
Reigniting hope in cancer treatment: the promise and pitfalls of IL-2 and IL-2R targeting strategies
Molecular Cancer (2023)
-
Regulatory T cells in autoimmune kidney diseases and transplantation
Nature Reviews Nephrology (2023)