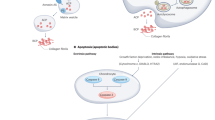
Key Points
-
Diabetes, metabolic syndrome and chronic infections increase glycaemia, lipidaemia and cellular levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS)
-
Increased glycaemia, lipidaemia, ROS and RNS induce glycation, glycoxidation, carbonylation and nitrosylation of cartilage proteins
-
Biochemical changes in proteins compromise the anatomical organization of cartilage
-
Changes in the anatomical organization of cartilage compromise tissue visco-elasticity and, ultimately, the ability of cartilage to sustain pressure and its overall performance
-
Protein biochemical changes are an early event in cartilage degeneration
Abstract
A hallmark of chronic metabolic diseases, such as diabetes and metabolic syndrome, and oxidative stress, as occurs in chronic inflammatory and degenerative conditions, is the presence of extensive protein post-translational modifications, including glycation, glycoxidation, carbonylation and nitrosylation. These modifications have been detected on structural cartilage proteins in joints and intervertebral discs, where they are known to affect protein folding, induce protein aggregation and, ultimately, generate microanatomical changes in the proteoglycan–collagen network that surrounds chondrocytes. Many of these modifications have also been shown to promote oxidative cleavage as well as enzymatically-mediated matrix degradation. Overall, a general picture starts to emerge indicating that biochemical changes in proteins constitute an early event that compromises the anatomical organization and viscoelasticity of cartilage, thereby affecting its ability to sustain pressure and, ultimately, impeding its overall bio-performance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Loeser, R. F. Aging processes and the development of osteoarthritis. Curr. Opin. Rheumatol. 25, 108–113 (2013).
Zhuo, Q., Yang, W., Chen, J. & Wang, Y. Metabolic syndrome meets osteoarthritis. Nat. Rev. Rheumatol. 8, 729–737 (2012).
Scharf, B. et al. Age-related carbonylation of fibrocartilage structural proteins drives tissue degenerative modification. Chem. Biol. 20, 922–934 (2013).
Goldring, M. B. Articular cartilage degradation in osteoarthritis. HSS J. 8, 7–9 (2012).
Buckwalter, J. A., Mankin, H. J. & Grodzinsky, A. J. Articular cartilage and osteoarthritis. Instr. Course Lect. 54, 465–480 (2005).
Wen, C. Y. et al. Collagen fibril stiffening in osteoarthritic cartilage of human beings revealed by atomic force microscopy. Osteoarthritis Cartilage 20, 916–922 (2012).
Martel-Pelletier, J., Lajeunesse, D., Fahmi, H., Tardif, G. & Pelletier, J. P. New thoughts on the pathophysiology of osteoarthritis: one more step toward new therapeutic targets. Curr. Rheumatol. Rep. 8, 30–36 (2006).
Bank, R. A., Bayliss, M. T., Lafeber, F. P., Maroudas, A. & Tekoppele, J. M. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem. J. 330, 345–351 (1998).
Fujioka, R., Aoyama, T. & Takakuwa, T. The layered structure of the articular surface. Osteoarthritis Cartilage 21, 1092–1098 (2013).
Wong, M. & Carter, D. R. Articular cartilage functional histomorphology and mechanobiology: a research perspective. Bone 33, 1–13 (2003).
Wilusz, R. E., Sanchez-Adams, J. & Guilak, F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 39, 25–32 (2014).
Eyre, D. R., Weis, M. A. & Wu, J. J. Articular cartilage collagen: an irreplaceable framework? Eur. Cell. Mater. 12, 57–63 (2006).
Gordon, M. K. & Hahn, R. A. Collagens. Cell Tissue Res. 339, 247–257 (2010).
Kiani, C., Chen, L., Wu, Y. J., Yee, A. J. & Yang, B. B. Structure and function of aggrecan. Cell Res. 12, 19–32 (2002).
Hardingham, T. E. & Fosang, A. J. Proteoglycans: many forms and many functions. FASEB J. 6, 861–870 (1992).
Hardingham, T. E., Fosang, A. J. & Dudhia, J. The structure, function and turnover of aggrecan, the large aggregating proteoglycan from cartilage. Eur. J. Clin. Chem. Clin. Biochem. 32, 249–257 (1994).
Aspberg, A. The different roles of aggrecan interaction domains. J. Histochem. Cytochem. 60, 987–996 (2012).
Han, E. H., Chen, S. S., Klisch, S. M. & Sah, R. L. Contribution of proteoglycan osmotic swelling pressure to the compressive properties of articular cartilage. Biophys. J. 101, 916–924 (2011).
Wilkins, R. J., Browning, J. A. & Ellory, J. C. Surviving in a matrix: membrane transport in articular chondrocytes. J. Membr. Biol. 177, 95–108 (2000).
Wilkins, R. J., Browning, J. A. & Urban, J. P. Chondrocyte regulation by mechanical load. Biorheology 37, 67–74 (2000).
Chubinskaya, S. et al. Response of human chondrocytes prepared for autologous implantation to growth factors. J. Knee Surg. 21, 192–199 (2008).
Mobasheri, A. The future of osteoarthritis therapeutics: emerging biological therapy. Curr. Rheumatol. Rep. 15, 385 (2013).
Verzijl, N. et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J. Biol. Chem. 275, 39027–39031 (2000).
Maroudas, A., Bayliss, M. T., Uchitel-Kaushansky, N., Schneiderman, R. & Gilav, E. Aggrecan turnover in human articular cartilage: use of aspartic acid racemization as a marker of molecular age. Arch. Biochem. Biophys. 350, 61–71 (1998).
Eyre, D. Collagen of articular cartilage. Arthritis Res. 4, 30–35 (2002).
Arokoski, J. P., Jurvelin, J. S., Vaatainen, U. & Helminen, H. J. Normal and pathological adaptations of articular cartilage to joint loading. Scand. J. Med. Sci. Sports 10, 186–198 (2000).
Kopesky, P. W. et al. Sustained delivery of bioactive TGF-β1 from self-assembling peptide hydrogels induces chondrogenesis of encapsulated bone marrow stromal cells. J. Biomed. Mater. Res. A 102, 1275–1285 (2014).
Houard, X., Goldring, M. B. & Berenbaum, F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr. Rheumatol. Rep. 15, 375 (2013).
Bucala, R. Diabetes, aging, and their tissue complications. J. Clin. Invest. 124, 1887–1888 (2014).
Stadtman, E. R. & Levine, R. L. Protein oxidation. Ann. N. Y. Acad. Sci. 899, 191–208 (2000).
Abramson, S. B. Osteoarthritis and nitric oxide. Osteoarthritis Cartilage 16 (Suppl. 2), S15–S20 (2008).
Cai, Z. & Yan, L. J. Protein oxidative modifications: beneficial roles in disease and health. J. Biochem. Pharmacol. Res. 1, 15–26 (2013).
Curtis, J. M. et al. A. Protein carbonylation and metabolic control systems. Trends Endocrinol. Metab. 23, 399–406 (2012).
Loeser, R. F., Gandhi, U., Long, D. L., Yin, W. & Chubinskaya, S. Aging and oxidative stress reduce the response of human articular chondrocytes to insulin-like growth factor-1 and osteogenic protein-1. Arthritis Rheumatol. 66, 2201–2209 (2014).
Hybertson, B. M., Gao, B., Bose, S. K. & McCord, J. M. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol. Aspects Med. 32, 234–246 (2011).
Berlett, B. S. & Stadtman, E. R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 272, 20313–20316 (1997).
Yan, L. J. Positive oxidative stress in aging and aging-related disease tolerance. Redox Biol. 2, 165–169 (2014).
Reddie, K. G. & Carroll, K. S. Expanding the functional diversity of proteins through cysteine oxidation. Curr. Opin. Chem. Biol. 12, 746–754 (2008).
Poole, L. B. & Nelson, K. J. Discovering mechanisms of signaling-mediated cysteine oxidation. Curr. Opin. Chem. Biol. 12, 18–24 (2008).
Hohn, A., Konig, J. & Grune, T. Protein oxidation in aging and the removal of oxidized proteins. J. Proteomics 92, 132–159 (2013).
Levine, R. L., Mosoni, L., Berlett, B. S. & Stadtman, E. R. Methionine residues as endogenous antioxidants in proteins. Proc. Natl Acad. Sci. USA 93, 15036–15040 (1996).
Arena, S., Salzano, A. M., Renzone, G., D'Ambrosio, C. & Scaloni, A. Non-enzymatic glycation and glycoxidation protein products in foods and diseases: an interconnected, complex scenario fully open to innovative proteomic studies. Mass Spectrom. Rev. 33, 49–77 (2014).
Tessier, F. J. The Maillard reaction in the human body. The main discoveries and factors that affect glycation. Pathol. Biol. (Paris) 58, 214–219 (2010).
Basle, E., Joubert, N. & Pucheault, M. Protein chemical modification on endogenous amino acids. Chem. Biol. 17, 213–227 (2010).
Vistoli, G. et al. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic. Res. 47 (Suppl. 1), 3–27 (2013).
Luevano-Contreras, C. & Chapman-Novakofski, K. Dietary advanced glycation end products and aging. Nutrients 2, 1247–1265 (2010).
Poulsen, M. W. et al. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 60, 10–37 (2013).
Baraibar, M. A., Liu, L., Ahmed, E. K. & Friguet, B. Protein oxidative damage at the crossroads of cellular senescence, aging, and age-related diseases. Oxid. Med. Cell Longev. 2012, 919832 (2012).
Terman, A. & Brunk, U. T. Aging as a catabolic malfunction. Int. J. Biochem. Cell Biol. 36, 2365–2375 (2004).
Grune, T., Merker, K., Jung, T., Sitte, N. & Davies, K. J. Protein oxidation and degradation during postmitotic senescence. Free Radic. Biol. Med. 39, 1208–1215 (2005).
Schett, G. et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes Care 36, 403–409 (2013).
Berenbaum, F. Diabetes-induced osteoarthritis: from a new paradigm to a new phenotype. Ann. Rheum. Dis. 70, 1354–1356 (2011).
Felson, D. T., Anderson, J. J., Naimark, A., Walker, A. M. & Meenan, R. F. Obesity and knee osteoarthritis. The Framingham Study. Ann. Intern. Med. 109, 18–24 (1988).
Pottie, P. et al. Obesity and osteoarthritis: more complex than predicted! Ann. Rheum. Dis. 65, 1403–1405 (2006).
Puenpatom, R. A. & Victor, T. W. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgrad. Med. 121, 9–20 (2009).
Cimmino, M. A. & Cutolo, M. Plasma glucose concentration in symptomatic osteoarthritis: a clinical and epidemiological survey. Clin. Exp. Rheumatol. 8, 251–257 (1990).
Nieves-Plaza, M., Castro-Santana, L. E., Font, Y. M., Mayor, A. M. & Vila, L. M. Association of hand or knee osteoarthritis with diabetes mellitus in a population of Hispanics from Puerto Rico. J. Clin. Rheumatol. 19, 1–6 (2013).
Engstrom, G., Gerhardsson de Verdier, M., Rollof, J., Nilsson, P. M. & Lohmander, L. S. C-reactive protein, metabolic syndrome and incidence of severe hip and knee osteoarthritis. A population-based cohort study. Osteoarthritis Cartilage 17, 168–173 (2009).
Velasquez, M. T. & Katz, J. D. Osteoarthritis: another component of metabolic syndrome? Metab. Syndr. Relat. Disord. 8, 295–305 (2010).
Mooney, R. A., Sampson, E. R., Lerea, J., Rosier, R. N. & Zuscik, M. J. High-fat diet accelerates progression of osteoarthritis after meniscal/ligamentous injury. Arthritis Res. Ther. 13, R198 (2011).
Griffin, T. M., Huebner, J. L., Kraus, V. B., Yan, Z. & Guilak, F. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: effects of short-term exercise. Arthritis Rheum. 64, 443–453 (2012).
Issa, R. I. & Griffin, T. M. Pathobiology of obesity and osteoarthritis: integrating biomechanics and inflammation. Pathobiol. Aging Age Relat. Dis. 2, 17470 (2012).
Kayal, R. A. et al. Diabetes causes the accelerated loss of cartilage during fracture repair which is reversed by insulin treatment. Bone 44, 357–363 (2009).
Atayde, S. A. et al. Experimental diabetes modulates collagen remodelling of joints in rats. Histol. Histopathol. 27, 1471–1479 (2012).
DeGroot, J. et al. Accumulation of advanced glycation endproducts reduces chondrocyte-mediated extracellular matrix turnover in human articular cartilage. Osteoarthritis Cartilage 9, 720–726 (2001).
Verzijl, N. et al. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem. J. 350, 381–387 (2000).
Weiss, R. E., Gorn, A. H. & Nimni, M. E. Abnormalities in the biosynthesis of cartilage and bone proteoglycans in experimental diabetes. Diabetes 30, 670–677 (1981).
DeGroot, J. et al. Accumulation of advanced glycation end products decreases collagen turnover by bovine chondrocytes. Exp. Cell Res. 266, 303–310 (2001).
DeGroot, J. et al. Age-related decrease in susceptibility of human articular cartilage to matrix metalloproteinase-mediated degradation: the role of advanced glycation end products. Arthritis Rheum. 44, 2562–2571 (2001).
Verzijl, N. et al. Age-related accumulation of the advanced glycation endproduct pentosidine in human articular cartilage aggrecan: the use of pentosidine levels as a quantitative measure of protein turnover. Matrix Biol. 20, 409–417 (2001).
Saudek, D. M. & Kay, J. Advanced glycation endproducts and osteoarthritis. Curr. Rheumatol. Rep. 5, 33–40 (2003).
Heywood, H. K., Nalesso, G., Lee, D. A. & Dell'accio, F. Culture expansion in low-glucose conditions preserves chondrocyte differentiation and enhances their subsequent capacity to form cartilage tissue in three-dimensional culture. Biores. Open Access 3, 9–18 (2014).
Vos, P. A. et al. Elevation of cartilage AGEs does not accelerate initiation of canine experimental osteoarthritis upon mild surgical damage. J. Orthop. Res. 30, 1398–1404 (2012).
Soltes, L. et al. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules 7, 659–668 (2006).
Hauselmann, H. J., Stefanovic-Racic, M., Michel, B. A. & Evans, C. H. Differences in nitric oxide production by superficial and deep human articular chondrocytes: implications for proteoglycan turnover in inflammatory joint diseases. J. Immunol. 160, 1444–1448 (1998).
Onur, T., Wu, R., Metz, L. & Dang, A. Characterisation of osteoarthritis in a small animal model of type 2 diabetes mellitus. Bone Joint Res. 3, 203–211 (2014).
Tsai, T. T. et al. Advanced glycation end products in degenerative nucleus pulposus with diabetes. J. Orthop. Res. 32, 238–244 (2014).
Mendoza, G. et al. Inhibitory effects of different antioxidants on hyaluronan depolymerization. Carbohydr. Res. 342, 96–102 (2007).
Taskiran, D., Stefanovic-Racic, M., Georgescu, H. & Evans, C. Nitric oxide mediates suppression of cartilage proteoglycan synthesis by interleukin-1. Biochem. Biophys. Res. Commun. 200, 142–148 (1994).
Bezerra, M. M. et al. Reactive nitrogen species scavenging, rather than nitric oxide inhibition, protects from articular cartilage damage in rat zymosan-induced arthritis. Br. J. Pharmacol. 141, 172–182 (2004).
Mosher, T. J. & Dardzinski, B. J. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin. Musculoskelet. Radiol. 8, 355–368 (2004).
Borthakur, A. & Reddy, R. Imaging cartilage physiology. Top. Magn. Reson. Imaging 21, 291–296 (2010).
Jungmann, P. M. et al. Association of metabolic risk factors with cartilage degradation assessed by T2 relaxation time at the knee: data from the osteoarthritis initiative. Arthritis Care Res. (Hoboken) 65, 1942–1950 (2013).
Athanasiou, K. A. et al. Effects of diabetes mellitus on the biomechanical properties of human ankle cartilage. Clin. Orthop. Relat. Res. 368, 182–189 (1999).
Verzijl, N. et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 46, 114–123 (2002).
Fick, J. M., Huttu, M. R., Lammi, M. J. & Korhonen, R. K. In vitro glycation of articular cartilage alters the biomechanical response of chondrocytes in a depth-dependent manner. Osteoarthritis Cartilage 22, 1410–1418 (2014).
Yin, W., Park, J. I. & Loeser, R. F. Oxidative stress inhibits insulin-like growth factor-I induction of chondrocyte proteoglycan synthesis through differential regulation of phosphatidylinositol 3-kinase-Akt and MEK-ERK MAPK signaling pathways. J. Biol. Chem. 284, 31972–31981 (2009).
Iwasa, K. et al. PTEN regulates matrix synthesis in adult human chondrocytes under oxidative stress. J. Orthop. Res. 32, 231–237 (2014).
DeGroot, J. et al. Accumulation of advanced glycation end products as a molecular mechanism for aging as a risk factor in osteoarthritis. Arthritis Rheum. 50, 1207–1215 (2004).
Legnani, C., Terzaghi, C., Borgo, E. & Ventura, A. Management of anterior cruciate ligament rupture in patients aged 40 years and older. J. Orthop. Traumatol. 12, 177–184 (2011).
Monnier, V. M. Intervention against the Maillard reaction in vivo. Arch. Biochem. Biophys. 419, 1–15 (2003).
Figarola, J. L. et al. LR-90 a new advanced glycation endproduct inhibitor prevents progression of diabetic nephropathy in streptozotocin-diabetic rats. Diabetologia 46, 1140–1152 (2003).
Steenvoorden, M. M. et al. Activation of receptor for advanced glycation end products in osteoarthritis leads to increased stimulation of chondrocytes and synoviocytes. Arthritis Rheum. 54, 253–263 (2006).
Loeser, R. F. et al. Articular chondrocytes express the receptor for advanced glycation end products: potential role in osteoarthritis. Arthritis Rheum. 52, 2376–2385 (2005).
Henrotin, Y. E., Bruckner, P. & Pujol, J. P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage 11, 747–755 (2003).
Rosa, S. C. et al. Impaired glucose transporter-1 degradation and increased glucose transport and oxidative stress in response to high glucose in chondrocytes from osteoarthritic versus normal human cartilage. Arthritis Res. Ther. 11, R80 (2009).
Abramson, S. B., Berenbaum, F., Hochberg, M. C. & Moskowitz, R. W. Introduction to OARSI FDA initiative OAC special edition. Osteoarthritis Cartilage 19, 475–477 (2011).
Sanchez-Adams, J., Leddy, H. A., McNulty, A. L., O'Conor, C. J. & Guilak, F. The mechanobiology of articular cartilage: bearing the burden of osteoarthritis. Curr. Rheumatol. Rep. 16, 451 (2014).
Grodzinsky, A. J., Levenston, M. E., Jin, M. & Frank, E. H. Cartilage tissue remodeling in response to mechanical forces. Annu. Rev. Biomed. Eng. 2, 691–713 (2000).
Lai, W. M., Hou, J. S. & Mow, V. C. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J. Biomech Eng. 113, 245–258 (1991).
Kempson, G. E., Muir, H., Pollard, C. & Tuke, M. The tensile properties of the cartilage of human femoral condyles related to the content of collagen and glycosaminoglycans. Biochim. Biophys. Acta 297, 456–472 (1973).
Williamson, A. K., Chen, A. C., Masuda, K., Thonar, E. J. & Sah, R. L. Tensile mechanical properties of bovine articular cartilage: variations with growth and relationships to collagen network components. J. Orthop. Res. 21, 872–880 (2003).
Armstrong, C. G. & Mow, V. C. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J. Bone Joint Surg. Am. 64, 88–94 (1982).
Temple, M. M., Xue, Y., Chen, M. Q. & Sah, R. L. Interleukin-1alpha induction of tensile weakening associated with collagen degradation in bovine articular cartilage. Arthritis Rheum. 54, 3267–3276 (2006).
Author information
Authors and Affiliations
Contributions
J.H. and L.S. researched the data for the article and wrote the manuscript. All authors (J.H., N.C. and L.S.) contributed substantially to discussions of the article content, and review or editing of manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Hardin, J., Cobelli, N. & Santambrogio, L. Consequences of metabolic and oxidative modifications of cartilage tissue. Nat Rev Rheumatol 11, 521–529 (2015). https://doi.org/10.1038/nrrheum.2015.70
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2015.70
This article is cited by
-
Highly bioavailable curcumin powder suppresses articular cartilage damage in rats with mono-iodoacetate (MIA)-induced osteoarthritis
Food Science and Biotechnology (2020)
-
Therapeutic “Tool” in Reconstruction and Regeneration of Tissue Engineering for Osteochondral Repair
Applied Biochemistry and Biotechnology (2020)
-
Anisotropy Properties of Tissues: A Basis for Fabrication of Biomimetic Anisotropic Scaffolds for Tissue Engineering
Journal of Bionic Engineering (2019)
-
Glycation marker glucosepane increases with the progression of osteoarthritis and correlates with morphological and functional changes of cartilage in vivo
Arthritis Research & Therapy (2018)
-
The interrelation of osteoarthritis and diabetes mellitus: considering the potential role of interleukin-10 and in vitro models for further analysis
Inflammation Research (2018)