Key Points
-
Worldwide incidence and prevalence estimates of systemic lupus erythematosus (SLE) vary substantially and are influenced by ethnic and geographic differences, study design and environmental exposures
-
Disease severity is greater in African American populations than in white populations
-
Poverty, low educational attainment, lack of health insurance, poor social support and poor treatment compliance are all associated with unfavourable disease outcomes, both independent of, and in combination with, ethnic influences
-
The treatment of SLE incurs high direct costs, and sometimes even higher indirect costs; costs are influenced by disease severity and organ manifestations
-
Health-related quality of life is greatly compromised in patients with SLE
Abstract
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease that can potentially lead to serious organ complications and even death. Its global burden — in terms of incidence and prevalence, differential impact on populations, economic costs and capacity to compromise health-related quality of life — remains incompletely understood. The reported worldwide incidence and prevalence of SLE vary considerably; this variation is probably attributable to a variety of factors, including ethnic and geographic differences in the populations being studied, the definition of SLE applied, and the methods of case identification. Despite the heterogeneous nature of the disease, distinct patterns of disease presentation, severity and course can often be related to differences in ethnicity, income level, education, health insurance status, level of social support and medication compliance, as well as environmental and occupational factors. Given the potential for the disease to cause such severe and widespread organ damage, not only are the attendant direct costs high, but these costs are sometimes exceeded by indirect costs owing to loss of economic productivity. As an intangible cost, patients with SLE are, not surprisingly, likely to endure considerably reduced health-related quality of life.
Similar content being viewed by others
Main
Systemic lupus erythematosus (SLE) is a systemic autoimmune rheumatic disease with a complex pathogenesis. SLE can potentially cause substantial physical and functional disability and its manifestations are tremendously diverse, ranging from relatively mild cutaneous and articular involvement through to debilitating fatigue, significant cognitive impairment, end-stage renal disease and catastrophic thrombosis1; hence, it is often called 'the disease of a thousand faces'. SLE is much more common in women than men, and some women with SLE have difficulty conceiving; many women with SLE — particularly those whose disease is active at the time of conception — also experience numerous complications, both maternal and fetal, during pregnancy2. Both men and women with SLE have a higher risk of developing cardiovascular and cerebrovascular disease and malignancy than individuals without SLE, as a consequence of both the disease and its treatments3,4. The treatment options for patients with SLE remain limited compared to those for other rheumatic diseases, such as rheumatoid arthritis, and existing therapies are ineffective or poorly tolerated in a sizeable proportion of patients. Unfortunately, almost all large-scale randomized trials of biologic therapies (with only one exception, belimumab) have failed to demonstrate efficacy in patients with SLE5. Consequently, progress in the treatment of SLE has been modest, with belimumab being the only new therapy approved in the last 50 years5.
As SLE is a relatively rare and complex disease, its global burden — in terms of incidence and prevalence, differential impact on populations, economic costs and capacity to compromise health-related quality of life — remains underappreciated and poorly understood. In this article, we first provide an overview of the worldwide incidence and prevalence of SLE, and discuss the factors that contribute to the considerable variation seen in these parameters. We then outline the factors known to contribute to health disparities relating to disease prevalence, development, manifestations and severity of SLE. Finally, we will examine the socioeconomic impact of SLE by detailing estimates and disease-related determinants of direct and indirect costs, and discussing the intangible costs reflected by impairments in health-related quality of life (HRQoL). By enhancing our understanding of the global burden of SLE and its determinants, this Review aims to inform future efforts to reduce health disparities and improve patient outcomes while optimizing resource allocation and decreasing associated health care costs.
The incidence and prevalence of SLE
Studies in the USA conducted between 1950 and 1992 reported an increasing incidence of SLE6. This increase was probably partially attributable to enhanced diagnostic capabilities — through technological advances in immunologic testing, increased awareness of SLE, the development of standardized classification criteria and greater access to specialty care — which enabled the identification of patients with mild SLE, in whom the diagnosis might previously have been missed7. Since these studies, some countries, such as Denmark and Norway, have reported a stable disease incidence8,9,10, whereas others, such as the UK and Greece, continue to report an increase7,11,12,13,14.
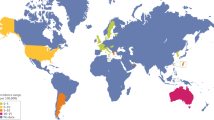
Reported values for the incidence and prevalence of SLE vary considerably worldwide (Box 1, Figs 1, 2, 3, Table 1, Supplementary information S1 (table)), with the overall incidence ranging from 0.3 per 100,000 per year in the Ukraine15 to 31.5 per 100,000 per year among Afro-Caribbean people living in the UK14, and the overall prevalence ranging from 3.2 per 100,000 in India16 to 517.5 per 100,000 among Afro-Caribbean people living in the UK14 (Supplementary information S1 (table)). Such variation might be caused by many factors involved in case identification and data collection, including whether the population studied was hospital-based or community- based, resided in a rural or an urban community, the structure of health-care delivery, the definition of SLE applied, the observation interval, and the method of case ascertainment.
Reported SLE prevalence ranges per country (per 100,000 of the population) are shown, as denoted by the key. The number of studies (n =) indicate those from which each prevalence range was determined. Note the dual shading of Spain, indicating that the prevalence values span two neighbouring ranges. Precise overall prevalence ranges per country are outlined in Table 1, in which data for Mainland China and Taiwan are listed independently. Nature Reviews Rheumatology remains neutral with regard to jurisdictional claims in published maps.
The figure shows the SLE prevalence ranges in women per country (per 100,000 of the population) as denoted by the key. The number of studies (n =) indicate those from which each prevalence range was determined. Precise female prevalence ranges per country are outlined in Table 1, in which data for Mainland China and Taiwan are listed independently. Nature Reviews Rheumatology remains neutral with regard to jurisdictional claims in published maps.
The figure shows the SLE prevalence ranges in men per country (per 100,000 of the population) as denoted by the key. The number of studies (n =) indicate those from which each prevalence range was determined. Precise male prevalence ranges per country are outlined in Table 1, in which data for Mainland China and Taiwan are listed independently. Nature Reviews Rheumatology remains neutral with regard to jurisdictional claims in published maps.
The most reliable incidence and prevalence data are likely to derive from studies in countries that have public health-care systems, in which patient care is centrally managed, national health insurance data are maintained, disease registries include only patients diagnosed by a specialist as having SLE, reliable census figures are available, and estimates of incidence and prevalence are determined over many years. Taiwan is one example of a country that fulfils these criteria. Nonetheless, the overall incidence of SLE varies from 4.9 to 9.9 per 100,000 per year and its overall prevalence from 37.0 to 97.5 per 100,000 in Taiwan17,18,19,20 (Supplementary information S1 (table)). Similar types of datum are available for other regions, but over shorter time periods21, including estimates from South Korea22,23, where the incidence of SLE ranges from 2.5 to 2.8 per 100,000 per year and its prevalence ranges from 20.6 to 26.5 per 100,000 (Supplementary information S1 (table)).
Two unique and particularly comprehensive strategies for case ascertainment in SLE are capture-recapture methods and the COPCORD (Community Oriented Program for Control of Rheumatic Diseases) approach. Capture-recapture methods use models to evaluate the completeness of case ascertainment by estimating the number of cases that are missed when multiple data sources are used for data analysis8,24,25,26. Two US studies using this methodology derived almost identical incidence and prevalence values: the Michigan Lupus Epidemiology and Surveillance Program24 reported an incidence of 5.5 per 100,000 per year and a prevalence of 72.8 per 100,000 and the Georgia Lupus Registry25 reported an incidence of 5.6 per 100,000 per year and a prevalence of 74.4 per 100,000. (Supplementary information S1 (table)) Other studies using these approaches provide incidence estimates of 1.0 per 100,000 per year in Denmark8 and prevalence estimates of 21.9–28.3 per 100,000 in Denmark8 and 25.4 per 100,000 in Ireland26 (Supplementary information S1 (table)).
COPCORD was devised as a low-cost method of determining the prevalence of various rheumatic diseases, including SLE27,28,29,30,31,32,33,34. This approach comprises three phases (screening, pre-evaluation and evaluation by a rheumatologist) utilizing local staff or health-care workers, and requires minimal use of investigations to determine diagnoses, which makes it a particularly appropriate tool for use in developing nations. Estimates of the prevalence of SLE derived using the COPCORD approach range from 0 per 100,000 among Turkish populations in Iran30, to 190.0 per 100,000 among white populations in Iran29, with estimates varying depending on the ethnic group and region studied. The number of studies using this approach continues to grow27,28,29,30,31,32,33,34.
Unfortunately, complete information, such as that obtained from comprehensive public health care systems with associated national registries, capture-recapture methods or the COPCORD approach, is not available for most nations, and other strategies for obtaining data (which probably provide less robust estimates) are applied. These strategies include identification of cases through hospital or community clinics in urban or rural areas6,11,15,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51, screening based on patient questionnaire responses or primary-care physician evaluations16,52,53,54, analysis of SLE trial cohort or registry population data8,12,55,56,57,58, review of private insurance or administrative databases (which might not include populations representative of the entire country13,14,19,23,59,60,61,62), or some combination of all of these approaches9,10,24,26,63,64. Community-based studies are likely to provide more accurate estimates than hospital-based ones, as the latter presumably include only patients with severe forms of SLE. Rural studies might also underestimate the incidence and prevalence of SLE, given the decreased access to specialist care in these areas. One study from the USA illustrates how prevalence estimates can vary depending on the definition of SLE used, with patient self-report of SLE diagnosis exceeding physician-confirmed diagnosis by threefold (372.0 versus 124.0 per 100,000)46. Methods that rely on data collected over a short time period (that is, weeks to months rather than years) will probably not generate meaningful estimates of incidence and prevalence, and studies that rely solely on medical claims data or physician billing codes, without having a medical chart review performed by a rheumatologist, could either underestimate or overestimate SLE incidence and prevalence. A US study based on Medicaid claims data65 reported a prevalence of 300.0 per 100,000, which is likely to represent an overestimate, as cases were not validated, and the sample was not representative of the general population — it included only low-income adults and their families, and patients with certain disabilities.
Patterns and trends
Despite the variations in the reported incidence and prevalence of SLE, definite trends have emerged. SLE typically presents between the ages of 15 and 45 years, with a 9:1 ratio of female to male patients66. Ethnic disparities are also widely recognized, with non-white populations generally having a higher incidence and prevalence of SLE compared to white populations; in the USA, the incidence and prevalence of SLE in African Americans is approximately twofold–fivefold higher than in European Americans24,25,45,59. In the UK, the prevalence of SLE is sixfold–eightfold higher in individuals of African ancestry and in Indo-Asian people than in white populations7,40,67,68,69. The disease is also twofold–fourfold more common among Aboriginal individuals compared to non-Aboriginal individuals living in Australia, Canada and the USA37,39,48,58,70,71. The incidence and prevalence of SLE is also higher in other populations that include individuals of African, Asian and Aboriginal ancestry9,12,14,15,17,24,25,35,37,39,40,45,48,58,59,71,72,73,74,75,76.
In contrast to the high prevalence of SLE seen in individuals of African ancestry living in Europe and North America, the prevalence of SLE in Africa itself has been thought to be quite low7. This observation generated the 'prevalence gradient' hypothesis, which suggests that the prevalence of SLE increases when people move to other nations from Africa, supporting the role of environmental triggers in disease development. However, although some evidence supports this hypothesis, no studies of the prevalence of SLE have been conducted in western Africa; the rarity of SLE has only been determined anecdotally, from case reports and case series7. In contrast to this hypothesis, evidence from the UK has shown a high prevalence of SLE among recent immigrants from West Africa, many of whom developed the disease before they immigrated40, and findings from Africa from the past decade indicate that SLE might not be as rare among African populations as previously thought7,77. These findings indicate that the apparently increased incidence and prevalence of SLE among people of African ancestry in Europe and North America might partly result from improved access to health care, which enables more patients to be diagnosed with SLE, as well as extending the survival of those living with SLE.
Clearly, many factors (primarily the methodologies used for case identification and data collection, the age, sex, and ethnic make-up of the population being studied, but also those contributing to the health disparities discussed in the next section) influence estimates of SLE incidence and prevalence. The global burden of SLE is, therefore, still not fully defined.
Health disparities
Health disparities are inequalities in health status among members of a given population. In addition to the differences in SLE incidence and prevalence already discussed, a range of other health disparities are well known to exist for SLE. Aspects of disease development, manifestations and severity are influenced by ethnicity, factors associated with socioeconomic status (including financial and educational status and levels of health insurance, social support and medication compliance) as well as by environmental and occupational exposures. All these factors can further influence the incidence and prevalence of SLE. Below we outline these varying influences (Table 2).
Ethnicity
Generally, patients of African ancestry and those from Asian, Hispanic and Aboriginal populations not only develop SLE earlier than do patients from white populations, but also tend to have a more acute disease onset, a greater number of (and more severe) clinical manifestations, higher disease activity and damage, and higher mortality9,12,14,15,17,24,25,35,37,39,40,45,48,58,59,71,72,73,74,75,76,78,79,80. Despite the fact that mortality from SLE has decreased significantly in the past few decades, it remains higher in Asian populations and patients of African ancestry than in white populations74,81,82,83,84, and deaths from SLE among African American women aged 45–64 years actually increased by almost 70% from 1979 to 1998 in the USA85. Survival in patients with SLE is shorter in parts of Asia and the developing world than in North America and Europe, which indicates the potential additional importance of environmental and other socioeconomic factors in the prognosis of SLE. However, we must acknowledge that not all studies take into consideration the baseline mortality in the general population when making these comparisons, and it is not always evident whether similar ethnic groups are being compared70,86.
Considerable evidence indicates that lupus nephritis is more prevalent in patients of African ancestry and Asian and Hispanic populations than in white populations9,24,25,35,56,59,67,79,86,87,88,89,90,91,92,93,94. Individuals of African ancestry accumulate more renal damage and are more likely to develop (and die from) end-stage renal disease than are those from white, Hispanic or Asian populations7,24,86,88,89,90,91,92,95,96. Although the majority of these comparative data originate from US studies, a single study also shows a greater incidence of lupus nephritis among Asian patients in Asia than among white patients in the USA94. These findings imply that, irrespective of their place of residence, Asian patients are more likely than white patients to develop renal disease, emphasizing the importance of ethnicity in the development of lupus nephritis.
Data regarding other organ manifestations of SLE are less definitive than those for lupus nephritis, but a few trends have emerged. Regardless of age and sex, Asian and Hispanic patients and those of African ancestry tend to have more haematologic, serologic and immunologic manifestations of SLE compared to white patie-nts24,25,70,73,79,80,82,87,88,90,97,98,99. Discoid lupus seems to be more common in patients of African ancestry than in white patients7,24,25,75,87,88,90,98, whereas white patients experience a higher frequency of photosensitivity and malar rash than do patients of African ancestry7,24,25,75,88,89,90,98. Indian patients with SLE tend to have an increased risk of neurological involvement7,35,82,88,100, whereas the evidence for patients of Asian, Hispanic and African ancestry is mixed7,72,78,79,80,82,89,90,96,97,101,102. However, disparities can also exist within ethnic groups: in the LUMINA (Lupus in Minorities: Nature Versus Nurture) study cohort, Puerto Rican Hispanic patients exhibited a higher frequency of cutaneous manifestations, less renal and neurological involvement, less disease activity and less organ damage than did Texan Hispanic patients (who were of Mexican or Central American ancestry)72,93,97, which further supports the possibility that environmental and socioeconomic influences are important.
Technological advances, including high-throughput genotyping and whole-genome sequencing, have contributed evidence for a role of genetic variation in SLE. Although an in-depth discussion of genetic variation in SLE is beyond the scope of this Review, many gene polymorphisms have been reported to be associated with disease manifestations, autoantibody profiles and clinical outcomes in patients with SLE, which might help to explain some, but not all, of the ethnic differences in disease presentation and progression80.
Socioeconomic status
On the basis of studies that have examined both genetic and socioeconomic factors in patients with SLE73,103, some researchers propose that genetic factors are most important at disease onset, whereas socioeconomic factors become more important over time75,88. Thus, differences in disease manifestations and course are likely to be the result of a complex interplay between genes and the environment, and it is often difficult to determine which factors predominate75. In many countries, socioeconomic status is highly related to ethnicity, with non-white individuals generally having a lower socioeconomic status than white people88,90,104. Low socioeconomic status has been associated with several adverse outcomes in patients with SLE, such as high disease activity, increased damage accrual, work disability and mortality59,68,72,75,79,88,89,99,105,106,107,108,109,110,111,112,113. As previously noted, the results of many studies show higher mortality among Asian, Hispanic and First Nations patients and those of African ancestry compared with white patients with SLE7,37,70,72,74,75,78,81,82,85,86,87,88,89,91,92,95,96,99,114. However, in some studies that adjusted for socioeconomic status, this difference was no longer observed70,72,90, suggesting that some health disparities are partially independent of ethnicity and so might be amenable to intervention. Stratification by socioeconomic status must be interpreted with caution115 as the results might be influenced not only by ethnicity but also by health outcomes, potentially creating a bias in the association observed between ethnic group and health outcomes. However, in a study of white female patients with SLE in the USA (in which the effects of ethnicity and socioeconomic status are not entangled owing to the focus on a single ethnic group) higher mortality was observed in women from areas with increased poverty114, emphasizing that socioeconomic status can have an important effect on mortality even in a subgroup that generally has a favourable prognosis.
Financial status and poverty
Several large cohort studies have demonstrated that poverty is associated with higher disease activity, increased organ damage and higher mortality in patients with SLE of varying ethnic backgrounds, compared to patients with SLE of higher financial status79,97,104,105,116,117,118,119. The LUMINA cohort, initiated in 1994, includes over 600 patients with SLE of white, Hispanic and African American ethnicity from Alabama, Texas and Puerto Rico72,73,97,99,116,117. LUMINA cohort studies demonstrated that patients with SLE living below the poverty line were four times more likely to die than those with incomes above this level97,116,117. The GLADEL (Grupo Latino Americano de Estudio de Lupus) cohort, started in 1997, includes almost 1,500 patients with SLE of mestizo, white or 'other' ethnicity, recruited from 34 centres in Latin America79. Increased organ damage (OR 1.4) and mortality were observed in those with low incomes, with 70.6% of the patients who died belonging to the lower and middle socioeconomic groups79. The Hopkins cohort, begun in 1987, includes over 2,000 patients with SLE, primarily white and African American, seen by one provider in the USA119; patients with an annual household income below $25,000 had an estimated 20-year survival of 70%, compared with 86% for those above this threshold118. Poverty has also been associated with lower mental functioning in patients with SLE105 and has been shown to contribute to the progression of lupus nephritis, independent of ethnicity120,121. Data from the USA have shown that high-income patients who develop end-stage renal disease secondary to lupus nephritis have improved survival, and that this association might counteract the mortality difference between African American and non-African American groups outlined above92.
Education
Multiple SLE studies, including those involving the LUMINA and GLADEL cohorts, have shown that low education levels are linked to high disease activity, increased organ damage, low physical functioning and high mortality79,104,105,116,117,122. Low educational attainment in patients is correlated with physician under-diagnosis of SLE in non-white (Asian, African American and Asian or Pacific Islander) populations, and might also influence patients' satisfaction with their care, as well as their compliance with treatment80,122. Generally, Hispanic and African American individuals in the USA have lower levels of education than their white counterparts, and might also have limited English language skills. These limitations can interfere with the ability of patients to understand practitioners and with the capacity of health workers to provide proper care104,123. However, a high educational level does not always correlate with improved disease outcomes, and this association can be modified by ethnicity. A population-based study found that although high levels of education among white patients with SLE were associated with reduced mortality, a similar association was not seen in African American patients or women of Asian or Pacific Islander ethnicity122.
Health insurance
Another factor that is closely related to financial status and education, and contributes to health disparities, is the ability to obtain medical insurance and to access health care resources. Lack of health insurance, which disproportionately affects non-white populations in the USA, might delay or prevent access to specialist rheumatology care, and can limit the treatment options available to patients123. In the USA, having private insurance has been linked to lower disease activity in patients with SLE of all ethnic groups, whereas public insurance, or lack of insurance, has been associated with increased disease activity, increased hospitalizations and increased mortality74,89,99. Financial status, educational level and health insurance are likely to act in synergy, rather than independently, to influence outcomes in SLE.
Social support and health perceptions
Social support is another component of a patient's socioeconomic status that modulates disease activity, damage accrual and level of functioning70,99,105. Adequate social support acts as a positive factor that enables patients and their families to better navigate, understand and use the health care system. Poor social support is associated with increased disease activity and impaired mental functioning, whereas lack of self-efficacy in disease management is associated with decreased mental and physical functioning99,105. These associations might also be affected by ethnic differences. For example, in the LUMINA cohort study, low levels of social support, high degrees of helplessness and poor coping styles were seen in Texan Hispanic and African American patients, but not in Puerto Rican Hispanic or white patients73,99,104.
Adverse health perceptions and maladaptive illness-related behaviours worsen disease outcomes, medication beliefs and compliance; they are also influenced by ethnicity80. Compared to white British patients with SLE, British patients of South Asian origin with SLE are more focused on the harmful effects (rather than the potential benefits) of prescription medications124. In consequence, patients of South Asian origin stop taking DMARDs sooner than do those of Northern European origin, owing to concerns regarding medication toxicity124. African American patients are also less willing than white American patients to take medications to treat SLE, and studies report decreased compliance in this ethnic group89,91,104.
Environmental and occupational factors
Smoking. Smoking is more common among individuals of low socioeconomic status than in other groups. Multiple studies have examined the relationship between smoking and SLE, with conflicting results68,125,126,127,128,129,130,131. Data on whether a dose–response relationship exists are also conflicting128. A 2015 meta-analysis showed that both former smoking and current smoking increase the risk of developing SLE, but this association was modified by geography; the risk of SLE was increased in current smokers in Europe and East Asia but not in North America, whereas the risk of developing SLE was increased in former smokers only in Europe126. Current smoking might also increase disease activity, with one study showing higher disease activity in current smokers compared to former or never smokers130. Furthermore, smoking might exacerbate the severity of organ involvement; some studies show increased pleuritis, peritonitis, neuropsychiatric symptoms and end-stage renal disease among smokers128,129,131.
Alcohol. The effect of alcohol on SLE is unclear. Alcohol consumption protects against the development of SLE in some, but not all, studies127,128,132,133,134,135. Some reports even suggest a dose–response relationship, with successive increments in alcohol consumption linked to further reductions in the risk of developing SLE127. A US case–control study found that, although current drinking levels were inversely associated with the risk of developing SLE, alcohol consumption before diagnosis showed no such correlation134. However, a tendency of patients to quit drinking just before or shortly after being diagnosed with SLE could partially explain these findings134. A meta-analysis of six case–control studies showed that a moderate alcohol intake protected against the development of SLE, but this association was less clear in a cohort study132,135. Many potential reasons might underlie these inconsistencies, including differences in the types and patterns of alcohol consumption, patient selection and recall bias, or uncontrolled confounding variables such as patients' educational levels. To date, no large, well-controlled, prospective cohort study has examined the association between long-term alcohol consumption and SLE risk. Thus, although the available data suggest that moderate alcohol consumption might be associated with a decreased risk of developing SLE, the evidence for this protective effect remains limited.
Silica. Case reports, case–control studies and observational cohorts have shown that exposure to silica is positively associated with the development of SLE136,137,138,139,140,141,142,143. Work in mines, quarries, foundries, roadways, construction, masonry, farming, sandblasting and the production of pottery, glass and tiles result in a relatively high exposure to silica. Cohort studies have demonstrated a tenfold higher risk of developing SLE in patients exposed to very high levels of silica compared to non-exposed patients141,142. Silica might also interact with other environmental factors to influence SLE development. A US case–control study suggested a possible interaction between silica and smoking, as the risk of developing SLE was increased in smokers who were exposed to high levels of silica, but not in smokers with low silica exposures140. By contrast, a Canadian case–control study found that silica-exposed people who had never smoked had an increased risk of developing SLE compared with never-smokers without silica exposure, but that silica exposure did not increase the risk of SLE in people who had ever smoked137. Overall, the balance of evidence seems to support the notion that silica exposure contributes to SLE development, but how smoking or other exposures might modify this risk remains unknown.
Industrial emissions, pollution, solvents and pesticides. Little research has evaluated the effects of pollution, solvents and pesticides on the development of SLE, but some evidence suggests that pollution does contribute to SLE development and disease activity136,144. A Canadian study showed that increased levels of air pollution were associated with increased disease activity, as reflected by the increased presence of urinary casts and levels of antibodies against double-stranded DNA145. Exposure to solvents has been associated with an increased risk of developing SLE, and with increased mortality137,146,147. A study conducted in Arizona also reported an increase in the number of symptoms in patients with SLE who had been exposed to solvent-contaminated well water148. By contrast, two case–control studies from the USA showed no increased risk of SLE in patients exposed to solvents143,147. Evidence regarding exposure to pesticides and the risk of developing SLE is conflicting68,139,146.
Vitamin D and sunlight or UV exposure. Vitamin D is believed to be an immune mediator, and multiple studies (although not all) have shown that patients with SLE have lower vitamin D levels than controls149,150,151,152,153. Low levels of sunlight exposure are linked to vitamin D deficiency; patients with SLE are, therefore, at a particularly high risk of vitamin D deficiency owing to photosensitivity and consequent sun avoidance. Moreover, their place of residence and ethnicity will further influence their vitamin D status150. However, whether low vitamin D levels are a consequence of SLE or have a causative role in its development is not clear. Animal models show that vitamin D deficiency contributes to the development of SLE, and that vitamin D supplements can reduce SLE symptoms150,154,155. However, the data are less definitive in humans. Some studies show that low vitamin D levels are associated with increased SLE disease activity150,151,153,156,157,158,159, but others (including one meta-analysis) do not support this relationship149,152,160,161. Furthermore, supplementation with vitamin D has shown benefit in some studies but not others149,156,162.
Conflicting evidence also exists regarding the role of UV exposure in the development of SLE. A UK study showed an increased disease incidence in northern regions of the UK, where UV levels become progressively lower with increasing latitudes163, whereas a study from Sweden reported that women with highly sun-sensitive skin types and patients who had experienced severe sunburn during their youth showed an increased risk of developing SLE133. Also unresolved is whether UV exposure improves or exacerbates existing SLE. A study from Hong Kong showed that most disease flares occurred in December and January164, whereas the Hopkins Cohort in the USA experienced exacerbations of arthritis and skin manifestations during the spring and summer165. Geographic patterns of SLE mortality in the USA have been suggested to be consistent with regional differences in UV levels, with one study reporting higher mortality among African American and white patients with SLE in areas with high levels of UV exposure114.
The burden of SLE
Economic implications
Understanding the economic burden of SLE is crucial to determining the optimal allocation of health care resources, with the ultimate goal of improving patients' outcomes. However, accurate calculation of disease-related costs is very challenging, and estimates must incorporate both direct (Box 2) and indirect (Box 3) costs. Direct costs are the value of resources used in the prevention, diagnosis, treatment and rehabilitation of a disease; indirect costs represent the value of economic productivity lost owing to disease-related disability in both labour and non-labour market activities166. Whereas assessments of health resource utilization can be based on patient self-reported data or review of medical charts or insurance databases, diminished productivity is almost always derived from patient self- reported data. Distinguishing which costs are attributable directly to SLE and which are attributable to comorbidities (which might not be associated with SLE) is difficult, so most cost-of-illness estimates for SLE incorporate all health resource utilization and diminished productivity, regardless of the cause.
Direct costs. Although SLE currently cannot be cured, survival and life expectancy have increased over the past few decades as a result of improvements in diagnosis and treatment. However, SLE can still cause considerable organ damage, potentially leading to high morbidity and mortality. As such, the direct costs of the disease can be substantial166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188 (Table 3, Supplementary information S2 (table)), reaching up to $71,334.00 per patient per year184, and are increased by the development of organ dysfunction such as lupus nephritis168,175,179,183,184,185,188, disease flares176,178,179, high disease activity174,177,185,187 and disease of long duration167 (Table 3, Supplementary information S2 (table)). Data from the USA indicate that the direct costs in patients with lupus nephritis exceed those in the general population by sixfold, and Canadian data show that direct costs are almost five times greater in patients with end-stage renal disease than in those without renal damage185 (Table 3, Supplementary information S2 (table)). Furthermore, studies from mainland China, Canada and the USA show that SLE patients who are experiencing flares and severe disease incur direct costs twofold–sixfold higher than in patients with mild disease who do not experience flares174,176,187 (Table 3, Supplementary information S2 (table)).
Indirect costs. Given the chronic and unpredictable nature of SLE, the disease can significantly impair the ability of patients to work108,109,189 and lead to high indirect costs167,168,169,171,172,185,190 (Supplementary information S2 (table)). As SLE primarily affects women, the indirect costs encountered through diminished non-labour market activities (such as childcare and household work) are also considerable, and several studies have shown that indirect costs actually exceed direct costs by twofold–fourfold167,168,169,171,172,185,190 (Supplementary information S2 (table)).
The inability to work profoundly affects both the individual and society. Work loss contributes to further indirect costs through decreased socialization, low self-esteem and the reduced ability to support dependents; limited access to employer benefits such as health insurance, child care and pension plans; and the inability to save financially for retirement88,108,109,191. Work disability is common in patients with SLE, with 15–51% of patients reporting cessation of employment 2–15 years after diagnosis107,110,112,192,193,194, and over 60% being unemployed after 20 years112,191,195. Employment rates among patients with SLE are substantially lower than in the general population196. Although many studies do not directly compare individuals with SLE to healthy controls, data from the USA show that the unemployment rate within 1 year of diagnosis is 26% in patients with SLE, compared to only 9% in controls111. In Germany, employment among patients with SLE is 17–47% lower than the population average, depending on disease duration and sex197.
Work limitations in SLE have been associated with a variety of demographic, disease and job-related factors, including older age, low educational attainment, African ancestry, poverty, prolonged disease duration, high disease activity and damage, fatigue, musculoskeletal manifestations, neurocognitive involvement, anxiety, depression, increased pain, and physically and cognitively demanding types of work75,88,107,108,109,110,112,191,192,193,195,198,199,200,201. Even if patients with SLE remain employed, disease flares, organ damage and general poor health can decrease their productivity as well as contribute to an increased risk of permanent disability. Many individuals with SLE are compelled to reduce their working hours, alter their jobs, take extended sick leave or eventually claim disability assistance107,112,189,191,194,195,199,200. These limitations extend beyond their effects on work duties; the same demographic and disease-related factors are associated with a decreased ability to perform daily activities including studying, carrying out housework, caring for children and participating in leisure activities194,198,200.
Intangible losses
Beyond the financial burden, intangible losses should also be considered, as SLE contributes to decreased HRQoL via a wide range of adverse psychosocial factors196. HRQoL is a measure of a patient's physical and functional health, and also provides a view of their social environment and psychological beliefs, which might influence their response to illness202. Patients with SLE experience a lower HRQoL than do the general population, and the reductions are similar to, or even exceed, those for other chronic diseases70,113,189,196,198. Patients with SLE report reductions in all aspects of HRQoL, including physical and mental health, vitality, pain, and social and emotional functioning196. HRQoL is influenced by a complicated interplay between disease and environmental factors, and determinants include disease manifestations, particularly fatigue, disease activity and damage accrual, as well as the patient's level of helplessness and ability to cope with the disease70,189,196. Multiple other symptoms have been associated with poor HRQoL, including depression, anxiety, pain, sleep disturbances, and neuropsychiatric and cutaneous manifestations113,189,196.
Surprisingly, clinical measures of disease activity and organ damage do not always correlate with HRQoL196. However, decreasing disease activity can improve HRQoL113,202. Older age, female sex, poverty, low educational attainment, lack of social support, and unemployment are also associated with decreased HRQoL. Additionally, poor HRQoL is a determinant of reduced treatment compliance202, and the 2010 EULAR guidelines for monitoring patients with SLE consequently recommend that HRQoL should be assessed at every clinic visit203. However, given the multiple challenges faced by clinicians treating these patients — including the need to address diverse and complex organ manifestations, considerable comorbidities and the adverse effects of numerous, and potentially toxic, therapies — it is likely that assessment of HRQoL is often neglected, thereby contributing to poorer outcomes and higher direct and indirect costs.
Conclusions
SLE is an extremely heterogeneous disease in terms of development, presentation, manifestations and severity, and its global burden remains incompletely understood. The incidence and prevalence of SLE vary considerably; this variation is likely to be partly attributable to ethnic and geographic differences, the definition of SLE applied, and the methods of case ascertainment. However, the extremely varied estimates for SLE incidence and prevalence make it difficult to apportion resources appropriately for patient care; this challenge emphasizes the importance of further research applying a consistent disease definition and using standardized methodologies to overcome the problem of obtaining accurate data, with the aim of improving patient outcomes through appropriate health care planning and resource allocation.
Substantial evidence indicates that SLE develops more frequently, has a more severe disease course, causes more organ damage and has a higher mortality among Asian and Aboriginal populations and individuals of African ancestry than in white individuals. Data also clearly show that socioeconomic disparities (such as poverty, low educational status, lack of health insurance and poor social support, which are generally more common among non-white populations) operate collectively, as well as independently, to negatively influence the course and outcomes of SLE.
Various environmental exposures, including cigarette smoking, silica, pollution and solvents, might also increase the risk of developing SLE and influence disease severity and manifestations. Whether alcohol intake confers a protective effect on SLE development remains controversial, and the contribution of vitamin D and UV radiation levels to the pathogenesis of SLE is also unclear. Given the many and diverse manifestations of SLE, the treatment of patients — particularly those with lupus nephritis — is likely to incur substantial direct and indirect costs, and the patients themselves can experience a considerably impaired HRQoL. By describing the global burden of SLE and its determinants, we hope that this article will help to inform efforts to reduce health disparities and improve outcomes in patients with SLE, while decreasing costs and increasing productivity.
Review criteria
Articles for inclusion in this Review were obtained through multiple PubMed searches conducted using the search term “lupus” in combination with the search terms “race”, “ethnicity”, “socioeconomic status”, “poverty”, “education”, “health insurance”, “social support”, “smoking”, “alcohol”, “silica”, “pollution”, “solvents”, “pesticides” and “vitamin D”. The resulting abstracts were then reviewed by a single individual. No date range was specified and only full-text studies in English were included. Any additional relevant references found in review articles or primary articles that met these criteria were also included.
References
Tsokos, G. C. Systemic lupus erythematosus. N. Engl. J. Med. 365, 2110–2121 (2011).
de Jesus, G. R. et al. Understanding and managing pregnancy in patients with lupus. Autoimmune Dis. 2015, http://dx.doi.org/10.1155/2015/943490 (2015).
Bernatsky, S. et al. Cancer risk in systemic lupus: an updated international multi-centre cohort study. J. Autoimmun. 42, 130–135 (2013).
Manzi, S. et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham study. Am. J. Epidemiol. 145, 408–415 (1997).
Aytan, J. & Bukhari, M. A. Use of biologics in SLE: a review of the evidence from a clinical perspective. Rheumatology (Oxford) 55, 775–779 (2016).
Uramoto, K. M. et al. Trends in the incidence and mortality of systemic lupus erythematosus, 1950–1992. Arthritis Rheum. 42, 46–50 (1999).
Borchers, A. T., Naguwa, S. M., Shoenfeld, Y. & Gershwin, M. E. The geoepidemiology of systemic lupus erythematosus. Autoimmun. Rev. 9, A277–A287 (2010).
Laustrup, H., Voss, A., Green, A. & Junker, P. Occurrence of systemic lupus erythematosus in a Danish community: an 8-year prospective study. Scand. J. Rheumatol. 38, 128–132 (2009).
Lerang, K., Gilboe, I., Garen, T., Thelle, D. S. & Gran, J. T. High incidence and prevalence of systemic lupus erythematosus in Norway. Lupus 21, 1362–1369 (2012).
Nossent, H. C. Systemic lupus erythematosus in the Arctic region of Norway. J. Rheumatol. 28, 539–546 (2001).
Alamanos, Y. et al. Epidemiology of systemic lupus erythematosus in northwest Greece 1982–2001. J. Rheumatol. 30, 731–735 (2003).
Hopkinson, N. D., Doherty, M. & Powell, R. J. The prevalence and incidence of systemic lupus erythematosus in Nottingham, UK, 1989–1990. Br. J. Rheumatol. 32, 110–115 (1993).
Nightingale, A. L., Farmer, R. D. & de Vries, C. S. Incidence of clinically diagnosed systemic lupus erythematosus 1992–1998 using the UK general practice research database. Pharmacoepidemiol. Drug Saf. 15, 656–661 (2006).
Rees, F. et al. The incidence and prevalence of systemic lupus erythematosus in the UK, 1999–2012. Ann. Rheum. Dis. 75, 136–141 (2016).
Nasonov, E. et al. The prevalence and incidence of systemic lupus erythematosus (SLE) in selected cities from three Commonwealth of Independent States countries (the Russian Federation, Ukraine and Kazakhstan). Lupus 23, 213–219 (2014).
Malaviya, A. N., Singh, R. R., Singh, Y. N., Kapoor, S. K. & Kumar, A. Prevalence of systemic lupus erythematosus in India. Lupus 2, 115–118 (1993).
Chiu, Y. M. & Lai, C. H. Nationwide population-based epidemiologic study of systemic lupus erythematosus in Taiwan. Lupus 19, 1250–1255 (2010).
Yeh, K. W., Yu, C. H., Chan, P. C., Horng, J. T. & Huang, J. L. Burden of systemic lupus erythematosus in Taiwan: a population-based survey. Rheumatol. Int. 33, 1805–1811 (2013).
Yu, K. H., See, L. C., Kuo, C. F., Chou, I. J. & Chou, M. J. Prevalence and incidence in patients with autoimmune rheumatic diseases: a nationwide population-based study in Taiwan. Arthritis Care Res. (Hoboken) 65, 244–250 (2013).
See, L. C., Kuo, C. F., Chou, I. J., Chiou, M. J. & Yu, K. H. Sex- and age-specific incidence of autoimmune rheumatic diseases in the Chinese population: a Taiwan population-based study. Semin. Arthritis Rheum. 43, 381–386 (2013).
Arnaud, L. et al. Prevalence and incidence of systemic lupus erythematosus in France: a 2010 nation-wide population-based study. Autoimmun. Rev. 13, 1082–1089 (2014).
Shim, J. S., Sung, Y. K., Joo, Y. B., Lee, H. S. & Bae, S. C. Prevalence and incidence of systemic lupus erythematosus in South Korea. Rheumatol. Int. 34, 909–917 (2014).
Ju, J. H. et al. Prevalence of systemic lupus erythematosus in South Korea: an administrative database study. J. Epidemiol. 24, 295–303 (2014).
Somers, E. C. et al. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan lupus epidemiology and surveillance program. Arthritis Rheumatol. 66, 369–378 (2014).
Lim, S. S. et al. The incidence and prevalence of systemic lupus erythematosus, 2002–2004: the Georgia Lupus Registry. Arthritis Rheumatol. 66, 357–368 (2014).
Gourley, I. S., Patterson, C. C. & Bell, A. L. The prevalence of systemic lupus erythematosus in Northern Ireland. Lupus 6, 399–403 (1997).
Farooqi, A. & Gibson, T. Prevalence of the major rheumatic disorders in the adult population of north Pakistan. Br. J. Rheumatol. 37, 491–495 (1998).
Moghimi, N. et al. WHO-ILAR COPCORD study (stage 1, urban study) in Sanandaj, Iran. Clin. Rheumatol. 34, 535–543 (2015).
Sandoughi, M. et al. Prevalence of musculoskeletal disorders in southeastern Iran: a WHO-ILAR COPCORD study (stage 1, urban study). Int. J. Rheum. Dis. 16, 509–517 (2013).
Davatchi, F. et al. Effect of ethnic origin (Caucasians versus Turks) on the prevalence of rheumatic diseases: a WHO-ILAR COPCORD urban study in Iran. Clin. Rheumatol. 28, 1275–1282 (2009).
Senna, E. R. et al. Prevalence of rheumatic diseases in Brazil: a study using the COPCORD approach. J. Rheumatol. 31, 594–597 (2004).
Granados, Y. et al. Prevalence of musculoskeletal disorders and rheumatic diseases in an urban community in Monagas State, Venezuela: a COPCORD study. Clin. Rheumatol. 34, 871–877 (2015).
Pelaez-Ballestas, I. et al. Epidemiology of the rheumatic diseases in Mexico. A study of 5 regions based on the COPCORD methodology. J. Rheumatol. Suppl. 86, 3–8 (2011).
Reyes-Llerena, G. A. et al. Community-based study to estimate prevalence and burden of illness of rheumatic diseases in Cuba: a COPCORD study. J. Clin. Rheumatol. 15, 51–55 (2009).
Wang, F., Wang, C. L., Tan, C. T. & Manivasagar, M. Systemic lupus erythematosus in Malaysia: a study of 539 patients and comparison of prevalence and disease expression in different racial and gender groups. Lupus 6, 248–253 (1997).
Al-Rawi, Z., Al-Shaarbaf, H., Al-Raheem, E. & Khalifa, S. J. Clinical features of early cases of systemic lupus erythematosus in Iraqui patients. Br. J. Rheumatol. 22, 165–171 (1983).
Bossingham, D. Systemic lupus erythematosus in the far north of Queensland. Lupus 12, 327–331 (2003).
Shand, A. W., Algert, C. S., March, L. & Roberts, C. L. Second pregnancy outcomes for women with systemic lupus erythematosus. Ann. Rheum. Dis. 72, 547–551 (2013).
Segasothy, M. & Phillips, P. A. Systemic lupus erythematosus in Aborigines and Caucasians in central Australia: A comparative study. Lupus 10, 439–444 (2001).
Molokhia, M., McKeigue, P. M., Cuadrado, M. & Hughes, G. Systemic lupus erythematosus in migrants from west Africa compared with Afro-Caribbean people in the UK. Lancet 357, 1414–1415 (2001).
Alonso, M. D. et al. Systemic lupus erythematosus in northwestern Spain: a 20-year epidemiologic study. Medicine (Baltimore) 90, 350–358 (2011).
Lopez, P., Mozo, L., Gutierrez, C. & Suarez, A. Epidemiology of systemic lupus erythematosus in a northern spanish population: gender and age influence on immunological features. Lupus 12, 860–865 (2003).
Govoni, M., Castellino, G., Bosi, S., Napoli, N. & Trotta, F. Incidence and prevalence of systemic lupus erythematosus in a district of north Italy. Lupus 15, 110–113 (2006).
Nived, O., Sturfelt, G. & Wollheim, F. Systemic lupus erythematosus in an adult population in southern Sweden: incidence, prevalence and validity of ARA revised classification criteria. Br. J. Rheumatol. 24, 147–154 (1985).
Fessel, W. J. Systemic lupus erythematosus in the community. incidence, prevalence, outcome, and first symptoms; the high prevalence in black women. Arch. Intern. Med. 134, 1027–1035 (1974).
Hochberg, M. C. et al. Prevalence of self-reported physician-diagnosed systemic lupus erythematosus in the USA. Lupus 4, 454–456 (1995).
Phan, J. C., Bush, T. M., Donald, F. & Ward, M. Clinical and laboratory features of patients of Vietnamese descent with systemic lupus erythematosus. Lupus 8, 521–524 (1999).
Barnabe, C. et al. Prevalence of systemic lupus erythematosus and systemic sclerosis in the First Nations population of Alberta, Canada. Arthritis Care Res. (Hoboken) 64, 138–143 (2012).
Bernatsky, S. et al. A population-based assessment of systemic lupus erythematosus incidence and prevalence — results and implications of using administrative data for epidemiological studies. Rheumatology (Oxford) 46, 1814–1818 (2007).
Gudmundsson, S. & Steinsson, K. Systemic lupus erythematosus in Iceland 1975 through 1984. A nationwide epidemiological study in an unselected population. J. Rheumatol. 17, 1162–1167 (1990).
Nossent, J. C. Systemic lupus erythematosus on the Caribbean island of Curaçao: an epidemiological investigation. Ann. Rheum. Dis. 51, 1197–1201 (1992).
Zou, Y. F. et al. Prevalence of systemic lupus erythematosus and risk factors in rural areas of Anhui province. Rheumatol. Int. 34, 347–356 (2014).
Cakir, N. et al. The prevalences of some rheumatic diseases in western Turkey: Havsa study. Rheumatol. Int. 32, 895–908 (2012).
Anagnostopoulos, I. et al. The prevalence of rheumatic diseases in central Greece: a population survey. BMC Musculoskelet. Disord. 11, http://dx.doi.org/10.1186/1471-2474-11-98 (2010).
Mok, C. C., To, C. H., Ho, L. Y. & Yu, K. L. Incidence and mortality of systemic lupus erythematosus in a southern Chinese population, 2000–2006. J. Rheumatol. 35, 1978–1982 (2008).
Iseki, K. et al. An epidemiologic analysis of end-stage lupus nephritis. Am. J. Kidney Dis. 23, 547–554 (1994).
Stahl-Hallengren, C., Jonsen, A., Nived, O. & Sturfelt, G. Incidence studies of systemic lupus erythematosus in southern Sweden: increasing age, decreasing frequency of renal manifestations and good prognosis. J. Rheumatol. 27, 685–691 (2000).
Ferucci, E. D. et al. Prevalence and incidence of systemic lupus erythematosus in a population-based registry of American Indian and Alaska Native people, 2007–2009. Arthritis Rheumatol. 66, 2494–2502 (2014).
Feldman, C. H. et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with medicaid coverage, 2000–2004. Arthritis Rheum. 65, 753–763 (2013).
Scolnik, M. et al. Incidence and prevalence of lupus in Buenos Aires, Argentina: an 11-year health management organisation-based study. Lupus Sci. Med. 1, http://dx.doi.org/10.1136/lupus-2014-000021 (2014).
Naleway, A. L., Davis, M. E., Greenlee, R. T., Wilson, D. A. & McCarty, D. J. Epidemiology of systemic lupus erythematosus in rural Wisconsin. Lupus 14, 862–866 (2005).
Sardu, C. et al. Population based study of 12 autoimmune diseases in Sardinia, Italy: prevalence and comorbidity. PLoS ONE 7, http://dx.doi.org/10.1371/journal.pone.0032487 (2012).
Balluz, L. et al. Investigation of systemic lupus erythematosus in Nogales, Arizona. Am. J. Epidemiol. 154, 1029–1036 (2001).
Vilar, M. J. & Sato, E. I. Estimating the incidence of systemic lupus erythematosus in a tropical region (Natal, Brazil). Lupus 11, 528–532 (2002).
Garris, C., Shah, M. & Farrelly, E. The prevalence and burden of systemic lupus erythematosus in a Medicare population: retrospective analysis of Medicare claims. Cost Eff. Resour. Alloc. 13, http://dx.doi.org/10.1186/s12962-015-0034-z (2015).
McMurray, R. W. & May, W. Sex hormones and systemic lupus erythematosus: review and meta-analysis. Arthritis Rheum. 48, 2100–2110 (2003).
Patel, M., Clarke, A. M., Bruce, I. N. & Symmons, D. P. The prevalence and incidence of biopsy-proven lupus nephritis in the UK: evidence of an ethnic gradient. Arthritis Rheum. 54, 2963–2969 (2006).
Molokhia, M. & McKeigue, P. Systemic lupus erythematosus: genes versus environment in high risk populations. Lupus 15, 827–832 (2006).
Molokhia, M. & McKeigue, P. Risk for rheumatic disease in relation to ethnicity and admixture. Arthritis Res. 2, 115–125 (2000).
Pons-Estel, G. J., Alarcon, G. S., Scofield, L., Reinlib, L. & Cooper, G. S. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin. Arthritis Rheum. 39, 257–268 (2010).
Vincent, F. B., Bourke, P., Morand, E. F., Mackay, F. & Bossingham, D. Focus on systemic lupus erythematosus in indigenous Australians: towards a better understanding of autoimmune diseases. Intern. Med. J. 43, 227–234 (2013).
Alarcon, G. S. et al. Systemic lupus erythematosus in three ethnic groups. IX. Differences in damage accrual. Arthritis Rheum. 44, 2797–2806 (2001).
Alarcon, G. S. et al. Systemic lupus erythematosus in three ethnic groups: II. features predictive of disease activity early in its course. LUMINA study group (lupus in minority populations, nature versus nurture). Arthritis Rheum. 41, 1173–1180 (1998).
Anderson, E., Nietert, P. J., Kamen, D. L. & Gilkeson, G. S. Ethnic disparities among patients with systemic lupus erythematosus in South Carolina. J. Rheumatol. 35, 819–825 (2008).
Gonzalez, L. A., Toloza, S. M., McGwin, G., Jr. & Alarcon, G. S. Ethnicity in systemic lupus erythematosus (SLE): its influence on susceptibility and outcomes. Lupus 22, 1214–1224 (2013).
Ong, C., Nicholls, K. & Becker, G. Ethnicity and lupus nephritis: an Australian single centre study. Intern. Med. J. 41, 270–278 (2011).
Adelowo, O. O. & Oguntona, S. A. Pattern of systemic lupus erythematosus among Nigerians. Clin. Rheumatol. 28, 699–703 (2009).
Atisha-Fregoso, Y., Jakez-Ocampo, J. & Llorente, L. Systemic lupus erythematosus in Hispanics. Autoimmunity. 44, 555–561 (2011).
Pons-Estel, B. A. et al. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “Hispanics”. Medicine (Baltimore) 83, 1–17 (2004).
Mok, M. Y. & Li, W. L. Do Asian patients have worse lupus? Lupus 19, 1384–1390 (2010).
Kaslow, R. A. High rate of death caused by systemic lupus erythematosus among U. S. residents of Asian descent. Arthritis Rheum. 25, 414–418 (1982).
Samanta, A. et al. High prevalence of systemic disease and mortality in Asian subjects with systemic lupus erythematosus. Ann. Rheum. Dis. 50, 490–492 (1991).
Nossent, J. C. Course and prognostic value of systemic lupus erythematosus disease activity index in black Caribbean patients. Semin. Arthritis Rheum. 23, 16–21 (1993).
Bernatsky, S. et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 54, 2550–2557 (2006).
Krishnan, E. & Hubert, H. B. Ethnicity and mortality from systemic lupus erythematosus in the US. Ann. Rheum. Dis. 65, 1500–1505 (2006).
Wadee, S., Tikly, M. & Hopley, M. Causes and predictors of death in South Africans with systemic lupus erythematosus. Rheumatology (Oxford) 46, 1487–1491 (2007).
Lau, C. S., Yin, G. & Mok, M. Y. Ethnic and geographical differences in systemic lupus erythematosus: An overview. Lupus 15, 715–719 (2006).
Gonzalez, L. A., Toloza, S. M. & Alarcon, G. S. Impact of race and ethnicity in the course and outcome of systemic lupus erythematosus. Rheum. Dis. Clin. North Am. 40, 433–454, (2014).
Petri, M., Perez-Gutthann, S., Longenecker, J. C. & Hochberg, M. Morbidity of systemic lupus erythematosus: role of race and socioeconomic status. Am. J. Med. 91, 345–353 (1991).
Ward, M. M. & Studenski, S. Clinical manifestations of systemic lupus erythematosus. identification of racial and socioeconomic influences. Arch. Intern. Med. 150, 849–853 (1990).
Korbet, S. M., Schwartz, M. M., Evans, J. Lewis, E. J. & Collaborative Study Group. Severe lupus nephritis: racial differences in presentation and outcome. J. Am. Soc. Nephrol. 18, 244–254 (2007).
Nee, R. et al. Survival disparity of African American versus non-African American patients with ESRD due to SLE. Am. J. Kidney Dis. 66, 630–637 (2015).
Burgos, P. I. et al. US patients of Hispanic and African ancestry develop lupus nephritis early in the disease course: data from LUMINA, a multiethnic US cohort (LUMINA LXXIV). Ann. Rheum. Dis. 70, 393–394 (2011).
Thumboo, J. et al. A comparative study of the clinical manifestations of systemic lupus erythematosus in Caucasians in Rochester, Minnesota, and Chinese in Singapore, from 1980 to 1992. Arthritis Rheum. 45, 494–500 (2001).
Gomez-Puerta, J. A. et al. Racial and ethnic differences in mortality and cardiovascular events among patients with end-stage renal disease due to lupus nephritis. Arthritis Care Res. (Hoboken) 67, 1453–1462 (2015).
Heller, T., Ahmed, M., Siddiqqi, A., Wallrauch, C. & Bahlas, S. Systemic lupus erythematosus in Saudi Arabia: morbidity and mortality in a multiethnic population. Lupus 16, 908–914 (2007).
Alarcon, G. S. et al. Systemic lupus erythematosus in three ethnic groups: III. A comparison of characteristics early in the natural history of the LUMINA cohort. Lupus in Minority Populations: Nature versus Nurture. Lupus 8, 197–209 (1999).
Cooper, G. S. et al. Differences by race, sex and age in the clinical and immunologic features of recently diagnosed systemic lupus erythematosus patients in the southeastern United States. Lupus 11, 161–167 (2002).
Alarcon, G. S. et al. Systemic lupus erythematosus in a multiethnic cohort: LUMINA XXXV. Predictive factors of high disease activity over time. Ann. Rheum. Dis. 65, 1168–1174 (2006).
Malaviya, A. N., Chandrasekaran, A. N., Kumar, A. & Shamar, P. N. Systemic lupus erythematosus in India. Lupus 6, 690–700 (1997).
Connelly, K., Morand, E. F. & Hoi, A. Y. Asian ethnicity in systemic lupus erythematosus: an Australian perspective. Intern. Med. J. 43, 618–624 (2013).
Johnson, S. R., Urowitz, M. B., Ibanez, D. & Gladman, D. D. Ethnic variation in disease patterns and health outcomes in systemic lupus erythematosus. J. Rheumatol. 33, 1990–1995 (2006).
Reveille, J. D. et al. Systemic lupus erythematosus in three ethnic groups: I. The effects of HLA class II, C4, and CR1 alleles, socioeconomic factors, and ethnicity at disease onset. LUMINA study group. Lupus in Minority Populations, Nature versus Nurture. Arthritis Rheum. 41, 1161–1172 (1998).
Vina, E. R., Masi, C. M., Green, S. L. & Utset, T. O. A study of racial/ethnic differences in treatment preferences among lupus patients. Rheumatology (Oxford) 51, 1697–1706 (2012).
Karlson, E. W. et al. The relationship of socioeconomic status, race, and modifiable risk factors to outcomes in patients with systemic lupus erythematosus. Arthritis Rheum. 40, 47–56 (1997).
Odutola, J. & Ward, M. M. Ethnic and socioeconomic disparities in health among patients with rheumatic disease. Curr. Opin. Rheumatol. 17, 147–152 (2005).
Al Dhanhani, A. M., Gignac, M. A., Su, J. & Fortin, P. R. Work disability in systemic lupus erythematosus. Arthritis Rheum. 61, 378–385 (2009).
Baker, K. & Pope, J. Employment and work disability in systemic lupus erythematosus: a systematic review. Rheumatology (Oxford) 48, 281–284 (2009).
Baker, K. et al. Work disability in systemic lupus erythematosus is prevalent and associated with socio-demographic and disease related factors. Lupus 18, 1281–1288 (2009).
Bertoli, A. M., Fernandez, M., Alarcon, G. S., Vila, L. M. & Reveille, J. D. Systemic lupus erythematosus in a multiethnic US cohort LUMINA (XLI): factors predictive of self-reported work disability. Ann. Rheum. Dis. 66, 12–17 (2007).
Campbell, R., Jr., Cooper, G. S. & Gilkeson, G. S. The impact of systemic lupus erythematosus on employment. J. Rheumatol. 36, 2470–2475 (2009).
Drenkard, C. et al. Burden of systemic lupus erythematosus on employment and work productivity: data from a large cohort in the southeastern United States. Arthritis Care Res. (Hoboken) 66, 878–887 (2014).
Lau, C. S. & Mak, A. The socioeconomic burden of SLE. Nat. Rev. Rheumatol. 5, 400–404 (2009).
Walsh, S. J. & Gilchrist, A. Geographical clustering of mortality from systemic lupus erythematosus in the United States: contributions of poverty, Hispanic ethnicity and solar radiation. Lupus 15, 662–670 (2006).
Richiardi, L., Bellocco, R. & Zugna, D. Mediation analysis in epidemiology: methods, interpretation and bias. Int. J. Epidemiol. 42, 1511–1519 (2013).
Alarcon, G. S. Lessons from LUMINA: a multiethnic US cohort. Lupus 17, 971–976 (2008).
Alarcon, G. S. et al. Systemic lupus erythematosus in three ethnic groups. XIV. Poverty, wealth, and their influence on disease activity. Arthritis Rheum. 51, 73–77 (2004).
Kasitanon, N., Magder, L. S. & Petri, M. Predictors of survival in systemic lupus erythematosus. Medicine (Baltimore) 85, 147–156 (2006).
Fangtham, M. & Petri, M. 2013 Update: Hopkins lupus cohort. Curr. Rheumatol. Rep. 15 http://dx.doi.org/10.1007/s11926-013-0360-0 (2013).
Burling, F. et al. Ethnic, clinical and immunological factors in systemic lupus erythematosus and the development of lupus nephritis: results from a multi-ethnic New Zealand cohort. Lupus 16, 830–837 (2007).
Barr, R. G. et al. Prognosis in proliferative lupus nephritis: the role of socio-economic status and race/ethnicity. Nephrol. Dial. Transplant. 18, 2039–2046 (2003).
Ward, M. M. Education level and mortality in systemic lupus erythematosus (SLE): evidence of underascertainment of deaths due to SLE in ethnic minorities with low education levels. Arthritis Rheum. 51, 616–624 (2004).
Escalante, A. & del Rincon, I. Epidemiology and impact of rheumatic disorders in the United States Hispanic population. Curr. Opin. Rheumatol. 13, 104–110 (2001).
Kumar, K. et al. Beliefs about medicines in patients with rheumatoid arthritis and systemic lupus erythematosus: a comparison between patients of South Asian and white British origin. Rheumatology (Oxford) 47, 690–697 (2008).
Costenbader, K. H. et al. Cigarette smoking and the risk of systemic lupus erythematosus: a meta-analysis. Arthritis Rheum. 50, 849–857 (2004).
Jiang, F., Li, S. & Jia, C. Smoking and the risk of systemic lupus erythematosus: an updated systematic review and cumulative meta-analysis. Clin. Rheumatol. 34, 1885–1892 (2015).
Hardy, C. J., Palmer, B. P., Muir, K. R., Sutton, A. J. & Powell, R. J. Smoking history, alcohol consumption, and systemic lupus erythematosus: a case–control study. Ann. Rheum. Dis. 57, 451–455 (1998).
Takvorian, S. U., Merola, J. F. & Costenbader, K. H. Cigarette smoking, alcohol consumption and risk of systemic lupus erythematosus. Lupus 23, 537–544 (2014).
Rubin, R. L. et al. Effect of cigarette smoke on autoimmunity in murine and human systemic lupus erythematosus. Toxicol. Sci. 87, 86–96 (2005).
Ghaussy, N. O., Sibbitt, W. Jr., Bankhurst, A. D. & Qualls, C. R. Cigarette smoking and disease activity in systemic lupus erythematosus. J. Rheumatol. 30, 1215–1221 (2003).
Ward, M. M. & Studenski, S. Clinical prognostic factors in lupus nephritis. The importance of hypertension and smoking. Arch. Intern. Med. 152, 2082–2088 (1992).
Wang, J., Pan, H. F., Ye, D. Q., Su, H. & Li, X. P. Moderate alcohol drinking might be protective for systemic lupus erythematosus: a systematic review and meta-analysis. Clin. Rheumatol. 27, 1557–1563 (2008).
Bengtsson, A. A., Rylander, L., Hagmar, L., Nived, O. & Sturfelt, G. Risk factors for developing systemic lupus erythematosus: a case–control study in southern Sweden. Rheumatology (Oxford) 41, 563–571 (2002).
Wang, J., Kay, A. B., Fletcher, J., Formica, M. K. & McAlindon, T. E. Alcohol consumption is not protective for systemic lupus erythematosus. Ann. Rheum. Dis. 68, 345–348 (2009).
Formica, M. K., Palmer, J. R., Rosenberg, L. & McAlindon, T. E. Smoking, alcohol consumption, and risk of systemic lupus erythematosus in the Black Women's Health study. J. Rheumatol. 30, 1222–1226 (2003).
Farhat, S. C. et al. Air pollution in autoimmune rheumatic diseases: a review. Autoimmun Rev. 11, 14–21 (2011).
Cooper, G. S. et al. Occupational and environmental exposures and risk of systemic lupus erythematosus: silica, sunlight, solvents. Rheumatology (Oxford) 49, 2172–2180 (2010).
Cooper, G. S., Miller, F. W. & Germolec, D. R. Occupational exposures and autoimmune diseases. Int. Immunopharmacol. 2, 303–313 (2002).
Parks, C. G. & Cooper, G. S. Occupational exposures and risk of systemic lupus erythematosus: a review of the evidence and exposure assessment methods in population- and clinic-based studies. Lupus 15, 728–736 (2006).
Parks, C. G. et al. Occupational exposure to crystalline silica and risk of systemic lupus erythematosus: a population-based, case–control study in the southeastern United States. Arthritis Rheum. 46, 1840–1850 (2002).
Conrad, K., Mehlhorn, J., Luthke, K., Dorner, T. & Frank, K. H. Systemic lupus erythematosus after heavy exposure to quartz dust in uranium mines: clinical and serological characteristics. Lupus 5, 62–69 (1996).
Sanchez-Roman, J., Wichmann, I., Salaberri, J., Varela, J. M. & Nunez-Roldan, A. Multiple clinical and biological autoimmune manifestations in 50 workers after occupational exposure to silica. Ann. Rheum. Dis. 52, 534–538 (1993).
Finckh, A. et al. Occupational silica and solvent exposures and risk of systemic lupus erythematosus in urban women. Arthritis Rheum. 54, 3648–3654 (2006).
Dahlgren, J. et al. Cluster of systemic lupus erythematosus (SLE) associated with an oil field waste site: a cross sectional study. Environ. Health 6, http://dx.doi.org/10.1186/1476-069X-6-8 (2007).
Bernatsky, S. et al. Associations between ambient fine particulate levels and disease activity in patients with systemic lupus erythematosus (SLE). Environ. Health Perspect. 119, 45–49 (2011).
Parks, C. G. & De Roos, A. J. Pesticides, chemical and industrial exposures in relation to systemic lupus erythematosus. Lupus 23, 527–536 (2014).
Cooper, G. S. et al. Occupational risk factors for the development of systemic lupus erythematosus. J. Rheumatol. 31, 1928–1933 (2004).
Kilburn, K. H. & Warshaw, R. H. Prevalence of symptoms of systemic lupus erythematosus (SLE) and of fluorescent antinuclear antibodies associated with chronic exposure to trichloroethylene and other chemicals in well water. Environ. Res. 57, 1–9 (1992).
Sahebari, M., Nabavi, N. & Salehi, M. Correlation between serum 25(OH)D values and lupus disease activity: an original article and a systematic review with meta-analysis focusing on serum VitD confounders. Lupus 23, 1164–1177 (2014).
Breslin, L. C., Magee, P. J., Wallace, J. M. & McSorley, E. M. An evaluation of vitamin D status in individuals with systemic lupus erythematosus. Proc. Nutr. Soc. 70, 399–407 (2011).
de Souza, V. A. et al. Association of hypovitaminosis D with systemic lupus erythematosus and inflammation. J. Bras. Nefrol. 36, 430–436 (2014).
Fragoso, T. S. et al. 25-hydroxyivitamin D3 levels in patients with systemic lupus erythematosus and its association with clinical parameters and laboratory tests. Rev. Bras. Reumatol. 52, 60–65 (2012).
Hamza, R. T., Awwad, K. S., Ali, M. K. & Hamed, A. I. Reduced serum concentrations of 25-hydroxy vitamin D in Egyptian patients with systemic lupus erythematosus: relation to disease activity. Med. Sci. Monit. 17, CR711–CR718 (2011).
Abe, J. et al. Prevention of immunological disorders in Mrl/l mice by a new synthetic analogue of vitamin D3: 22-oxa-1α, 25-dihydroxyvitamin D3. J. Nutr. Sci. Vitaminol. (Tokyo) 36, 21–31 (1990).
Lemire, J. M., Ince, A. & Takashima, M. 1,25-dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of Mrl/l mice. Autoimmunity 12, 143–148 (1992).
Yap, K. et al. Association of low vitamin D with high disease activity in an Australian systemic lupus erythematosus cohort. Lupus Sci. Med. 2, http://dx.doi.org/10.1136/lupus-2014-000064 (2015).
Mok, C. C. et al. Vitamin D deficiency as marker for disease activity and damage in systemic lupus erythematosus: a comparison with anti-dsDNA and anti-C1q. Lupus 21, 36–42 (2012).
McGhie, T. K., DeCeulaer, K., Walters, C. A., Soyibo, A. & Lee, M. G. Vitamin D levels in Jamaican patients with systemic lupus erythematosus. Lupus 23, 1092–1096 (2014).
Barbhaiya, M. & Costenbader, K. H. Ultraviolet radiation and systemic lupus erythematosus. Lupus 23, 588–595 (2014).
Schneider, L. et al. Vitamin D levels and cytokine profiles in patients with systemic lupus erythematosus. Lupus 24, 1191–1197 (2015).
Munoz-Ortego, J., Torrente-Segarra, V., Prieto-Alhambra, D., Salman-Monte, T. C. & Carbonell-Abello, J. Prevalence and predictors of vitamin D deficiency in non-supplemented women with systemic lupus erythematosus in the Mediterranean region: a cohort study. Scand. J. Rheumatol. 41, 472–475 (2012).
Schneider, L., Dos Santos, A. S., Santos, M., da Silva Chakr, R. M. & Monticielo, O. A. Vitamin D and systemic lupus erythematosus: state of the art. Clin. Rheumatol. 33, 1033–1038 (2014).
Somers, E. C., Thomas, S. L., Smeeth, L., Schoonen, W. M. & Hall, A. J. Incidence of systemic lupus erythematosus in the United Kingdom, 1990–1999. Arthritis Rheum. 57, 612–618 (2007).
Szeto, C. C. et al. Climatic influence on the prevalence of noncutaneous disease flare in systemic lupus erythematosus in Hong Kong. J. Rheumatol. 35, 1031–1037 (2008).
Duarte-Garcia, A., Fang, H., To, C. H., Magder, L. S. & Petri, M. Seasonal variation in the activity of systemic lupus erythematosus. J. Rheumatol. 39, 1392–1398 (2012).
Panopalis, P. et al. Health care costs and costs associated with changes in work productivity among persons with systemic lupus erythematosus. Arthritis Rheum. 59, 1788–1795 (2008).
Huscher, D. et al. Cost of illness in rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and systemic lupus erythematosus in Germany. Ann. Rheum. Dis. 65, 1175–1183 (2006).
Jonsen, A. et al. Total cost and cost predictors in systemic lupus erythematosus — 8-years follow-up of a Swedish inception cohort. Lupus 24, 1248–1256 (2015).
Bexelius, C., Wachtmeister, K., Skare, P., Jonsson, L. & Vollenhoven, R. Drivers of cost and health-related quality of life in patients with systemic lupus erythematosus (SLE): a Swedish nationwide study based on patient reports. Lupus 22, 793–801 (2013).
Doria, A. et al. Annual direct medical cost of active systemic lupus erythematosus in five European countries. Ann. Rheum. Dis. 73, 154–160 (2014).
Sutcliffe, N., Clarke, A. E., Taylor, R., Frost, C. & Isenberg, D. A. Total costs and predictors of costs in patients with systemic lupus erythematosus. Rheumatology (Oxford) 40, 37–47 (2001).
Cho, J. et al. Costs of illness and quality of life in patients with systemic lupus erythematosus in South Korea. Lupus 23, 949–957 (2014).
Zhu, T. Y., Tam, L. S., Lee, V. W., Lee, K. K. & Li, E. K. Systemic lupus erythematosus with neuropsychiatric manifestation incurs high disease costs: a cost-of-illness study in Hong Kong. Rheumatology (Oxford) 48, 564–568 (2009).
Zhu, T. Y., Tam, L. S., Lee, V. W., Lee, K. K. & Li, E. K. The impact of flare on disease costs of patients with systemic lupus erythematosus. Arthritis Rheum. 61, 1159–1167 (2009).
Li, T. et al. Long-term medical costs and resource utilization in systemic lupus erythematosus and lupus nephritis: a five-year analysis of a large Medicaid population. Arthritis Rheum. 61, 755–763 (2009).
Garris, C. et al. Healthcare utilization and cost of systemic lupus erythematosus in a US managed care health plan. J. Med. Econ. 16, 667–677 (2013).
Kan, H. et al. A longitudinal analysis of costs associated with change in disease activity in systemic lupus erythematosus. J. Med. Econ. 16, 793–800 (2013).
Kan, H. J. et al. Healthcare utilization and costs of systemic lupus erythematosus in Medicaid. Biomed. Res. Int. 2013, http://dx.doi.org/10.1155/2013/808391 (2013).
Narayanan, S., Wilson, K., Ogelsby, A., Juneau, P. & Durden, E. Economic burden of systemic lupus erythematosus flares and comorbidities in a commercially insured population in the United States. J. Occup. Environ. Med. 55, 1262–1270 (2013).
Furst, D. E. et al. Medical costs and healthcare resource use in patients with lupus nephritis and neuropsychiatric lupus in an insured population. J. Med. Econ. 16, 500–509 (2013).
Furst, D. E. et al. Resource utilization and direct medical costs in adult systemic lupus erythematosus patients from a commercially insured population. Lupus 22, 268–278 (2013).
Oglesby, A. et al. Impact of early versus late systemic lupus erythematosus diagnosis on clinical and economic outcomes. Appl. Health Econ. Health Policy 12, 179–190 (2014).
Pelletier, E. M., Ogale, S., Yu, E., Brunetta, P. & Garg, J. Economic outcomes in patients diagnosed with systemic lupus erythematosus with versus without nephritis: results from an analysis of data from a US claims database. Clin. Ther. 31, 2653–2664 (2009).
Carls, G. et al. Direct and indirect costs to employers of patients with systemic lupus erythematosus with and without nephritis. J. Occup. Environ. Med. 51, 66–79 (2009).
Clarke, A. E. et al. SLE patients with renal damage incur higher health care costs. Rheumatology (Oxford) 47, 329–333 (2008).
Clarke, A. E. et al. The systemic lupus erythematosus Tri-Nation Study: absence of a link between health resource use and health outcome. Rheumatology (Oxford) 43, 1016–1024 (2004).
Clarke, A. E., Urowitz, M. B., Monga, N. & Hanly, J. G. Costs associated with severe and nonsevere systemic lupus erythematosus in Canada. Arthritis Care Res. (Hoboken) 67, 431–436 (2015).
Aghdassi, E. et al. Healthcare cost and loss of productivity in a Canadian population of patients with and without lupus nephritis. J. Rheumatol. 38, 658–666 (2011).
Gordon, C. et al. The substantial burden of systemic lupus erythematosus on the productivity and careers of patients: a European patient-driven online survey. Rheumatology (Oxford) 52, 2292–2301 (2013).
Panopalis, P. et al. The systemic lupus erythematosus Tri-Nation study: cumulative indirect costs. Arthritis Rheum. 57, 64–70 (2007).
Scofield, L., Reinlib, L., Alarcon, G. S. & Cooper, G. S. Employment and disability issues in systemic lupus erythematosus: a review. Arthritis Rheum. 59, 1475–1479 (2008).
Mok, C. C., Cheung, M. Y., Ho, L. Y., Yu, K. L. & To, C. H. Risk and predictors of work disability in Chinese patients with systemic lupus erythematosus. Lupus 17, 1103–1107 (2008).
Yelin, E. et al. Work loss and work entry among persons with systemic lupus erythematosus: comparisons with a national matched sample. Arthritis Rheum. 61, 247–258 (2009).
Zhu, T. Y., Tam, L. S. & Li, E. K. Labour and non-labour market productivity in Chinese patients with systemic lupus erythematosus. Rheumatology (Oxford) 51, 284–292 (2012).
Yelin, E. et al. Work dynamics among persons with systemic lupus erythematosus. Arthritis Rheum. 57, 56–63 (2007).
Schmeding, A. & Schneider, M. Fatigue, health-related quality of life and other patient-reported outcomes in systemic lupus erythematosus. Best Pract. Res. Clin. Rheumatol. 27, 363–375 (2013).
Mau, W., Listing, J., Huscher, D., Zeidler, H. & Zink, A. Employment across chronic inflammatory rheumatic diseases and comparison with the general population. J. Rheumatol. 32, 721–728 (2005).
Utset, T. O. et al. Work disability, lost productivity and associated risk factors in patients diagnosed with systemic lupus erythematosus. Lupus Sci. Med. 2, http://dx.doi.org/10.1136/lupus-2014-000058 (2015).
Jetha, A. et al. Unpacking early work experiences of young adults with rheumatic disease: an examination of absenteeism, job disruptions and productivity loss. Arthritis Care Res. (Hoboken) 67, 1246–1254 (2015).
Garris, C., Oglesby, A., Sulcs, E. & Lee, M. Impact of systemic lupus erythematosus on burden of illness and work productivity in the United States. Lupus 22, 1077–1086 (2013).
Al Dhanhani, A. M., Gignac, M. A., Beaton, D. E., Su, J. & Fortin, P. R. Work factors are associated with workplace activity limitations in systemic lupus erythematosus. Rheumatology (Oxford) 53, 2044–2052 (2014).
Dua, A. B., Touma, Z., Toloza, S. & Jolly, M. Top 10 recent developments in health-related quality of life in patients with systemic lupus erythematosus. Curr. Rheumatol. Rep. 15, http://dx.doi.org/10.1007/s11926-013-0380-9 (2013).
Mosca, M. et al. European league against rheumatism recommendations for monitoring patients with systemic lupus erythematosus in clinical practice and in observational studies. Ann. Rheum. Dis. 69, 1269–1274 (2010).
Author information
Authors and Affiliations
Contributions
A.E.C. and E.E.C. researched data for the article, contributed substantially to discussions of its content, and wrote the manuscript. All authors (A.E.C., E.E.C and S.G.B) undertook review and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
A.A.C. declares that she has served as a consultant for Astra-Zeneca, Bristol–Myers Squibb and Eli Lilly. The other authors declare no competing interests.
Supplementary information
Supplementary information S1 (table)
Worldwide studies of SLE prevalence and incidence (PDF 161 kb)
Supplementary information S2 (table)
Worldwide studies of SLE costs (PDF 565 kb)
Rights and permissions
About this article
Cite this article
Carter, E., Barr, S. & Clarke, A. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol 12, 605–620 (2016). https://doi.org/10.1038/nrrheum.2016.137
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2016.137
This article is cited by
-
Neddylation is a novel therapeutic target for lupus by regulating double negative T cell homeostasis
Signal Transduction and Targeted Therapy (2024)
-
The Impact of Systemic Lupus Erythematosus Flares on Clinical and Economic Outcomes: The CHAMOMILE Claims Database Study in Germany
Rheumatology and Therapy (2024)
-
Bibliometric analysis of global literature productivity in systemic lupus erythematosus from 2013 to 2022
Clinical Rheumatology (2024)
-
Association between uveitis onset and economic development in mainland China
BMC Public Health (2023)
-
m6A methyltransferase METTL3 programs CD4+ T-cell activation and effector T-cell differentiation in systemic lupus erythematosus
Molecular Medicine (2023)