Abstract
Background
As of 2014, the US FDA was considering policy options to promote accurate attribution of adverse events for biosimilars. In order to assess the identification and traceability of biologics from multiple sources, Tufts University’s Center for the Study of Drug Development conducted a study reviewing the current FDA Adverse Event Reporting System (FAERS) for reports related to insulin and growth hormone products.
Methods
For this study, all primary suspect reports that were received by FAERS for human growth hormone (hGH) and human insulin between the fourth quarter of 2005 and the third quarter of 2013 were extracted and analyzed.
Results
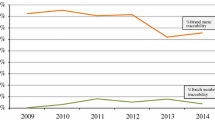
The rates of “accurate” brand (ie, identifiable) drug names were generally high, with a higher incidence for hGH drugs than for insulin drugs (92% of hGH primary suspect reports vs 84% of insulin primary suspect reports). Lot number completion rates were generally low, with a higher incidence for insulin drugs than for hGH drugs (37% of insulin primary suspect reports vs 13% of hGH primary suspect reports). There were 13.5% of insulin reports that could not be linked to manufacturers, while 7.5% of hGH reports could not be linked to a manufacturer.
Conclusions
The completion and accuracy rates of FAERS data on biologics observed in this study are consistent with those observed in earlier studies and suggest that traceability in adverse event reports can be improved through more consistent use of brand names or other product specific identifiers and through more frequent inclusion of lot numbers.
Similar content being viewed by others
References
Food and Drug Administration. Biologics Price Competition and Innovation Act of 2009. Washington, DC: Food Drug Administration; 2009.
Grabowski HG, Guha R, Salgado M. Regulatory and cost barriers are likely to limit biosimilar development and expected savings in the near future. Health Aff. 2014;33(6):1048–1057.
Pro Pharma Communications International. FDA Accepts Biosimilar Filgrastim Application: Generics and Biosimilars Initiative. Mol, Belgium: Pro Pharma Communications International; 2014.
Felix T, Johansson TT, Colliatie JA, Goldberg MR, Fox AR. Biologic product identification and US pharmacovigilance in the biosimilars era. Nat Biotech. 2014;32(2):128–130.
Pro Pharma Communications International. Hospira Submits Application to FDA for Epoetin Alfa Biosimilar. Mol, Belgium: Pro Pharma Communications International; 2015.
Pro Pharma Communications International. Apotex Pegfilgrastim Biosimilar Under FDA Review. Mol, Belgium: Pro Pharma Communications International; 2015.
Gaffney A. Sandoz First Company to File for Biosimilar Approval in US Under New Pathway. Rockville, MD: Regulatory Affairs Professionals Society; 2014.
Fitton PR. Comments of Amgen Inc on GPhA and Novartis’s Citizen Petitions Requesting Identical Non-proprietary Names for Biological Products and Their Respective Reference Products (Docket Nos FDA-2013-P-1153; FDA-2013-P-1398, Respectively. Thousand Oaks, CA: Amgen; 2013.
Carroll J. Manufacturers square off over naming of biosimilars. http://www.managedcaremag.com/linkout/2013/12/6. Published December 2013.
Fitton J. Biosimilar naming decision nears as applications arrive at FDA: the PRM report 2014. https://www.pharmamedtechbi.com/publications/rpm-report/10/9/biosimilar-naming-decision-nears-as-applications-arrive-at-fda. Accessed October 9, 2014.
Casadevall N, Felix T, Strober B, Warnock D. Similar names for similar biologics. BioDrugs. 2014;28(5):439–444.
Karlin S. Remicade biosimilar from celltrion includes bridging, extrapolation and switching data. https://www.pharmamedtechbi.com/publications/the-pink-sheet-daily/2014/8/11/emremicadeem-biosimilar-from-celltrion-includes-bridging-extrapolation-and-switching-data. Published August 11, 2014.
European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP) Guideline on Similar Biological Medicinal Products. London, England: European Medicines Agency; 2005.
Kozlowski S, Woodcock J, Midthun K, Behrman Sherman R. Developing the nation’s biosimilars program. N Engl J Med. 2011;365(5):385–388.
Medicines and Healthcare Products Regulatory Agency. Drug safety update: biosimilar products. http://www.mhra.gov.uk/Safetyinformation/DrugSafetyUpdate/CON084739. Published February 2008. Accessed October 9, 2014.
European Union. Directives: Commission Implementing Directive 2012/52/EU of 20 December 2012 Laying Down Measures to Facilitate the Recognition of Medical Prescriptions Issued in Another Member State. Brussels, Belgium: European Union; 2012.
Sandoz Limited. Binocrit solution for injection in a pre-filled syringe. http://www.medicines.org.uk/emc/history/21625/SPC/Binocrit+Solution+for+Injection+in+a+pre-filled+syringe. Accessed November 27, 2014.
European Union. Directive 2010/84/EU of the European Parliament and of the Council of 15 December 2010 Amending, as Regards Pharmacovigilance, Directive 2001/83/EC on the Community Code Relating to Medicinal Products for Human Use. Strasbourg, France: European Union; 2010.
Grampp G, Li E, Bonafede M, Felix T, Malecki M, Sprafka M. Traceability of Generic Enoxaparin in Active and Passive Surveillance Systems: Implications for Biosimilars Pharmacovigilance. Anaheim, CA: American Society of Health-System Pharmacists; 2014.
Cauchi R. State laws and legislation related to biologic medications and substitution of biosimilars. http://www.ncsl.org/research/health/state-laws-and-legislation-related-to-biologic-medications-and-substitution-of-biosimilars.aspx. Published August 4, 2014. Accessed October 9, 2014.
Lietzan EF, Sim LE, Alexander EA. FDLI’s Food and Drug Policy Form—Biosimilar Naming: How do Adverse Event Reporting Data Support the Need for Distinct Nonproprietary Names for Biosimilars? Washington, DC: Food and Drug Law Institute; 2013.
Vermeer NS, Straus SM, Mantel-Teeuwisse AK, et al. Traceability of biopharmaceuticals in spontaneous reporting systems: a cross-sectional study in the FDA Adverse Event Reporting System (FAERS) and EudraVigilance databases. Drug Saf. 2013;36(8):617–625.
Getz KA, Stergiopoulos S, Kaitin KI. Evaluating the completeness and accuracy of MedWatch data. Am J Ther. 2014;21(6):442–446.
Calvo B, Zuñiga L. EU’s new pharmacovigilance legislation: considerations for biosimilars. Drug Saf. 2014;37(1):9–18.
Food and Drug Administration. Substance registration system: unique ingredient identifier (UNII). http://fdasis.nlm.nih.gov/srs/srs.jsp. Published October 2014. Access October 9, 2014.
American Diabetes Association. Insulin administration. Diabetes Care. 2003;26(suppl 1):4.
Grimberg A, Feudtner C, Gordon C. Consequences of brand switches during the course of pediatric growth hormone treatment. Endocr Pract. 2012;18(3):307–216.
Sandoz Limited. Zarzio SmPC update. http://www.medicines.org.uk/emc/history/22310/SPC/Zarzio+30+MU+0.5+ml+solution+for+injection+or+infusion+in+pre+filled+syringe. Published 2011. Accessed November 27, 2013.
Vermeer NS, Spierings I, Mantel-Teeuwisse AK, et al. Traceability of biologicals: present challenges in pharmacovigilance. Expert Opin Drug Saf. 2015;14(1):63–72.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stergiopoulos, S., Brown, C.A., Grampp, G. et al. Identifying and Quantifying the Accuracy of Product Name Attribution of US-Sourced Adverse Event Reports in MedWatch of Somatropins and Insulins. Ther Innov Regul Sci 49, 706–716 (2015). https://doi.org/10.1177/2168479015578156
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1177/2168479015578156